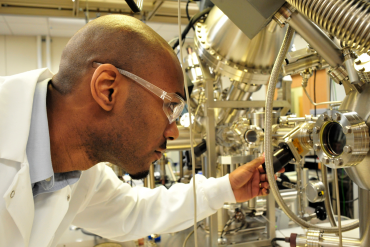
The U.S. Department of Energy and the U.S. lead battery industry are currently collaborating on an initiative to unlock the full potential of lead battery technology. The research project occurring at Argonne National Laboratory (ANL) in Chicago, is using the Advanced Photon Source (APS) – one of the world’s premier high-energy synchrotrons – to study electrochemical reactions in real time.
We had a chance to visit ANL and speak with materials scientist Dr. Tim Fister about the exciting progress being made. [NOTE: The original conversation has been condensed.]

Q. Explain the research. Initially, it began with RSR Technologies and East Penn Manufacturing, and then grew, correct?
Dr. Fister: Yes. They came to Argonne and were interested in understanding the origins and causes of sulfation in lead acid batteries. We developed a lot of interesting techniques [using the] APS to study these processes while operating the battery. We’ve recently renewed that effort and are now focusing on additives and dopants. But we’ve [added] 12 other [participating companies], looking at how lead sulfate forms when you turn on your car, when you discharge your battery on a model lead surface.
We’re exploring how these lead sulfate particles grow, their morphology, their size, their shape, and how it varies depending on how you run the battery, both the way that you apply the current and also the chemical environments that these particles are growing in. We’re using this baseline knowledge to revisit some of the design rules that are present in the lead acid battery and … figure out new ways to run these batteries.
Q. It’s early in the project, but have you had any surprising insights yet?
Dr. Fister: There’s a really rich phase space in the morphology of the lead sulfate particles that do form when you discharge the battery, and this can depend … on the ways that you discharge the battery. The current density, for instance, can also depend on the chemical conditions around the lead sulfate. So the concentration of acid … plays a distinct role in how these things form. What we found by watching these processes as the particles are growing and dissolving is contrary to popular wisdom. Sometimes the smaller particles are actually growing a bit more slowly and are sort of the problem species in these batteries. That’s been … surprising.
Q. Lead batteries have been around for 160 years. Don’t we already know everything about them? Aren’t they tapped out?
Dr. Fister: It’s a popular misconception that lead acid batteries are basically as good as they’ll ever be. When you look at the theoretical energy density and the specific capacity of these batteries, we’re actually only using a small fraction of the actual mass that’s in these batteries. And while that might be seen as a negative, I think it’s really exciting. There’s a lot of room for growth in this particular battery chemistry … it’s something that would really make it even more competitive for things like stationary storage.
Q. Do you think this research has the potential to increase the performance of the batteries?
Dr. Fister: Yes, I do. I can give you an example. Sometimes, when we’re watching these batteries at the APS, we find that certain parts of the battery are incredibly active, are essentially carrying the load for the entire battery cell and other parts of the battery are essentially dead. Understanding [this] … can help provide useful feedback to battery engineers for designing better cells down the road.
Q. How does your research differ from previous research on lead batteries?
Dr. Fister: There’s been an incredible amount of knowledge generated about lead acid batteries, but there hasn’t been that much done studying what’s happening inside a battery as you’re running it. One of the big, new innovations with this research is being able to watch the different species that are present, the morphology of the battery as you’re cycling it. Because, frankly, a lead acid battery changes. If you stop it, it can self-discharge. If you have to take it apart and rinse it off, by the time you take it to your microscope or whatever analytical tool you’re using, it could be different than when it’s actually running. I think that’s where we’ve brought some new insights.
Q. Are there other elements of a lead battery that should be investigated to better understand the battery or to explore potential impacts on performance?
Dr. Fister: Until now, we’ve been focusing largely on model lead surfaces and both surfaces of both electrodes. An interesting interface that we might … look at … may actually be the adhesion between the underlying grid, the current collector with the act of material itself. Frankly, for the lifetime of the batteries, this is often the part … that eventually fails.
Q. What’s the most interesting aspect of your work at Argonne?
Dr. Fister: Argonne is a huge place. There are a few thousand scientists here, and they come from all sorts of different backgrounds. For instance, my group is split into half electrochemists and half geochemists, which seems like kind of a funny mix, but we’re both interested in how solid liquid interfaces evolve by changing the conditions of the saturation and the electrolyte and stuff like that. That’s one of the rate-limiting steps in the lead acid batteries, the growth, in this case, of a lead sulfate mineral. Some of the recent advances in the growth models for how these crystals grow and dissolve is actually coming from that community [geochemists] more than the battery community. At Argonne, you can really get some novel insights from a wide range of people.
Q. How would you describe the relationship you’re developing with scientists and people in the U.S. lead battery community?
Dr. Fister: The interactions with the industry folks … has been refreshingly direct. It’s one of the few industry projects I’ve been involved in where people have flown out to help with the experiments. That has really accelerated the progress. I didn’t expect to be looking at real batteries at this point, and we’ve already started looking at motorcycle batteries from East Penn.
Q. You are part of a new generation of scientific researchers in the U.S. that can help the country maintain its leadership and fundamental research. Are you encouraged? How does your work in lead batteries contribute?
Dr. Fister: I feel quite lucky to be part of this generation of scientists in the U.S. There’s been some tremendous advances on my end, definitely from the light sources like the APS, but also computation power, the new supercomputers that are coming online, and some of the algorithms for analyzing these giant datasets that we collect. This is all happening, basically, as we speak. It’s an exciting time as we learn how to harness that level of complexity in our datasets.
Q. Can you explain how the APS helps your research?
Dr. Fister: I’ve been using the APS for nearly 15 years, and it’s crucial for my research. You can think of it as an extension … of the analytical tools we use in the lab. For instance, many battery companies use powder diffraction to analyze the different crystalline species that are present in their battery. APS has an x-ray beam that’s about a million times brighter than what you would see from a typical tube source. So what would normally be a static measurement in the lab is now a very dynamic measurement. We can actually measure things as species are growing and dissolving.
The APS is [also] incredibly focused. That allows us to look at things with spatial resolutions, sometimes as fine as 20 nanometers. And most importantly, APS is one of the few synchrotrons in the world that has a really high energy spectrum of x-rays. That’s critical when you work with lead batteries. We use lead as shielding, but to actually go through a lead acid battery, we can do that at some of the beamlines present here at the APS. That’s been eye-opening.
Q. What can you say about the new APS that’s going to be built. Will it impact your work?
Dr. Fister: The nearly billion-dollar APS upgrade is largely to replace the existing magnet lattice. The upgrade will essentially condense the electron beam, the x-rays that come off of it down to more or less a point source, so the resolution is going to be tremendous when you’re doing imaging type studies.The new lattice is [also] going to make the x-rays coherent. In a lead acid battery, you’re often dealing with particles that are changing shape, and we would be able to actually watch how these different crystal shapes are evolving … which crystal plans are active as you cycle the battery. I’m really excited about that new capability.
Q. Your research is pre-commercial. How does that contribute to innovation?
Dr. Fister: Pre-competitive research for us is really important, especially at the early stages of the research, because we need to go out and … discuss this research with our industrial collaborators freely. We need to go to conferences. We need to talk about this among the scientific community in general, just to generate ideas and come up with new ways to actually look at … these sorts of batteries.
Q. How do you define innovation?
Dr. Fister: I think innovation often happens as sort of non-linearities in the progress of how things happen. Oftentimes, innovation comes from approaching a problem with a fresh perspective. In the context of lead acid batteries, a lot of the process knowledge that we’ve accumulated … has had an inertia to it. So bringing in people, new scientists from places like Argonne, can really give us a fresh perspective on how these batteries actually operate. I’m hoping that these new takes on a relatively old chemistry will actually lead to some pretty abrupt innovations.