About Lead Batteries
Today’s innovative lead acid batteries are key to a cleaner, greener future and the foundation of our industry. They’re also the most environmentally sustainable battery technology and a stellar example of a circular economy.
Today’s innovative lead acid batteries are key to a cleaner, greener future and the foundation of our industry. They’re also the most environmentally sustainable battery technology and a stellar example of a circular economy.

Lead batteries play a critical role in powering everyday life and essential infrastructure. They provide reliable energy to start vehicles, support transportation systems, protect data and communication networks, and deliver backup power for hospitals, utilities, and emergency services. Without this proven technology, many of the systems people rely on daily would not function.
Used across transportation, industrial, and stationary applications, lead batteries support passenger and commercial vehicles, renewable energy storage, telecommunications, data centers, and critical facilities. Their durability, affordability, and recyclability make lead batteries an essential component of a resilient and sustainable energy ecosystem.
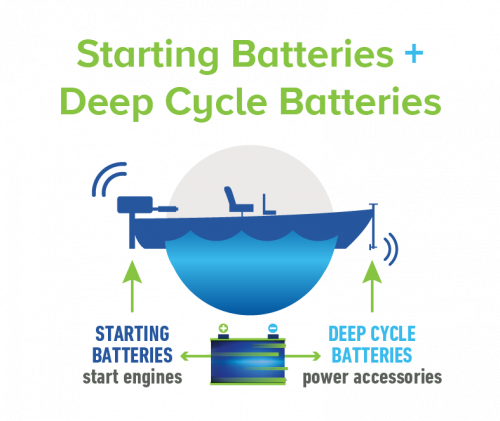
When people think about lead acid batteries, they usually think about a car battery. These are starting batteries. They deliver a short burst of high power to start the engine.
There are also deep cycle batteries. These are found on boats or campers, where they’re used to power accessories like trolling motors, winches or lights. They deliver a lower, steady level of power for a much longer time than a starting battery.
Lead batteries are used for a vast number of purposes, but all batteries provide either starting or deep cycle power. The only difference is how much power is delivered and how long it needs to be delivered.
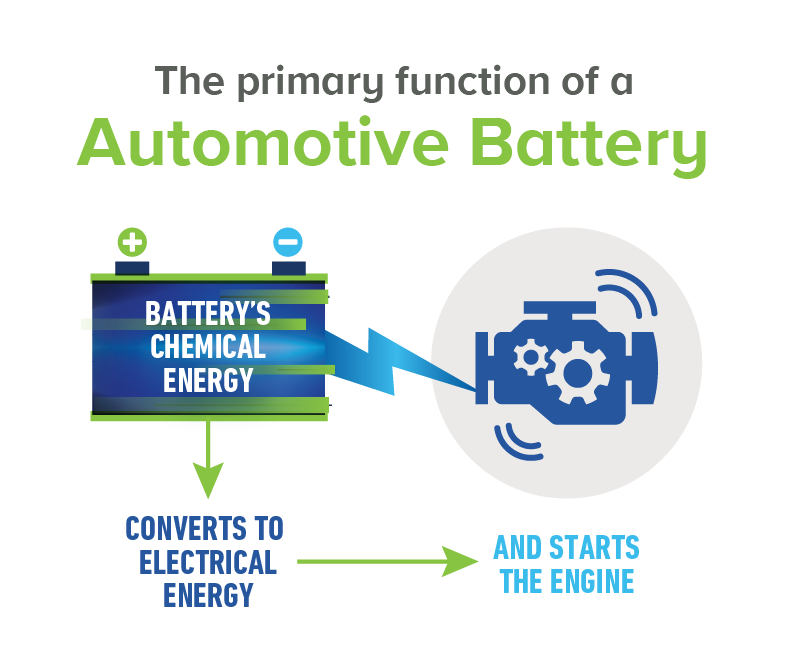
A car battery supplies power to the starter and ignition system to start the engine. They also supply the extra power necessary when the vehicle’s electrical load exceeds the supply from the charging system. A car battery acts as a voltage stabilizer in the electrical system. The battery evens out voltage spikes and prevents them from damaging other components in the electrical system.
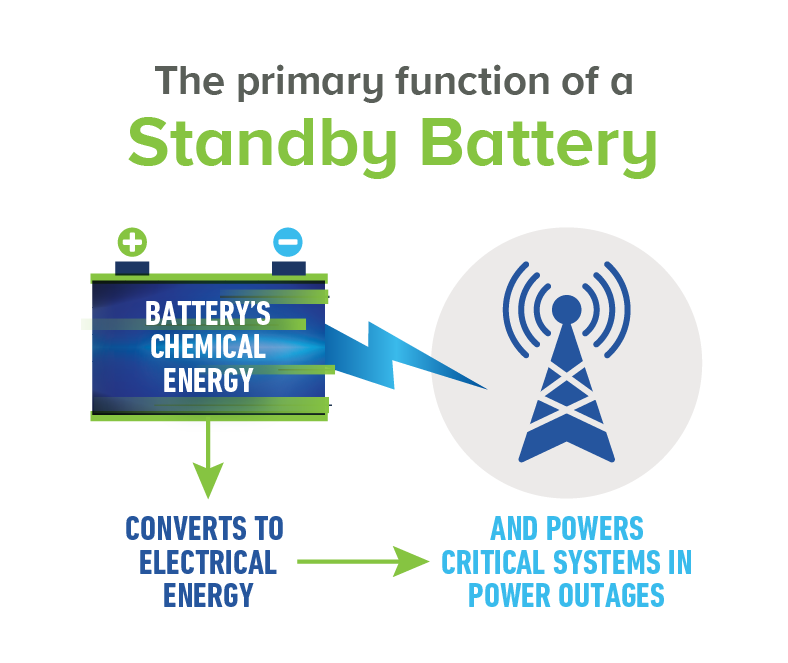
Standby batteries supply electrical power to critical systems in the event of a power outage. Hospitals, telecommunications systems, emergency lighting systems and many more rely on lead standby batteries to keep us safe without skipping a beat when the lights go out. Standby batteries are voltage stabilizers that smooth out fluctuations in electrical generation systems. These batteries temporarily hold large electrical loads as electric utilities switch from one generator system to another and can be extremely useful in times of need.
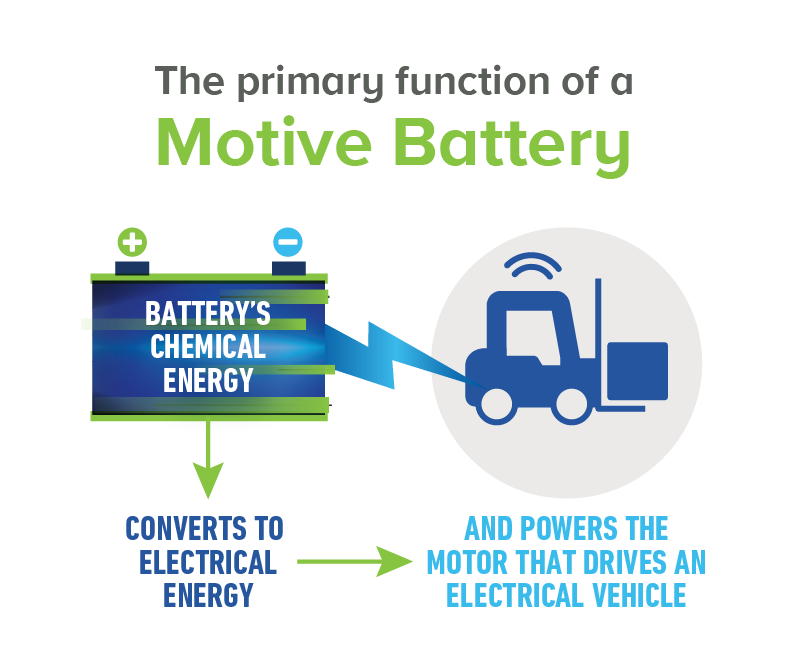
A motive battery powers the motor that drives an electric vehicle, such as a forklift truck; they also provide power for a specific purpose on an electric vehicle, such as the lift on a forklift truck. Additionally, motive batteries power accessories like headlights on an electric vehicle.
Small consumer sealed lead batteries are six-volt batteries that provide extended cycling service. Consumer sealed batteries power many consumer products, such as drills, flashlights, children’s toys and electric starters for gas lawn mowers.
Starting batteries are used to start engines on cars, boats and many other vehicles. Starting batteries provide a short burst of strong power in order to start the engine.
Deep cycle batteries power various electrical accessories, such as lights, trolling motors or winches, and motorized wheelchairs. Deep cycle batteries provide a low, steady level of power for a longer duration than a starting battery.
Dual purpose batteries are designed to serve a balanced combination of starting and deep cycle service. Dual purpose batteries have a high starting power for engine cranking but are able to withstand the cycle service demands from multiple accessory loads. Historically, most recreational vehicle (RV) and marine batteries have been designed to serve the dual purpose of starting engines and providing lighting and ignition (SLI) and also providing limited deep cycle capability for ‘house loads’ when the engine is not running. More recently, over the road trucks (18-wheelers) have increased their deep cycling requirements to provide deep cycling capability to limit the amount of time idling the engine when the driver has reached his legal driving time limit.
Industrial batteries, such as cell tower back-up power, provide a low, steady power for a longer duration than a typical deep cycle battery. The plates are thicker, and there is a higher amount of total energy available for a longer period of time. Industrial batteries have the ability to last for years and can be used in stationary applications that provide critical back-up power to systems that need constant power supply. Industrial batteries are often not called upon to deliver power, but when they are, it is required that they deliver an abundance of power that will last long enough for reserve generators to take over. Often times, industrial batteries are configured as systems to accommodate large power demands.
Nearly all lead batteries are made of recycled lead and plastic, and all are recycled at the end of their service lives.
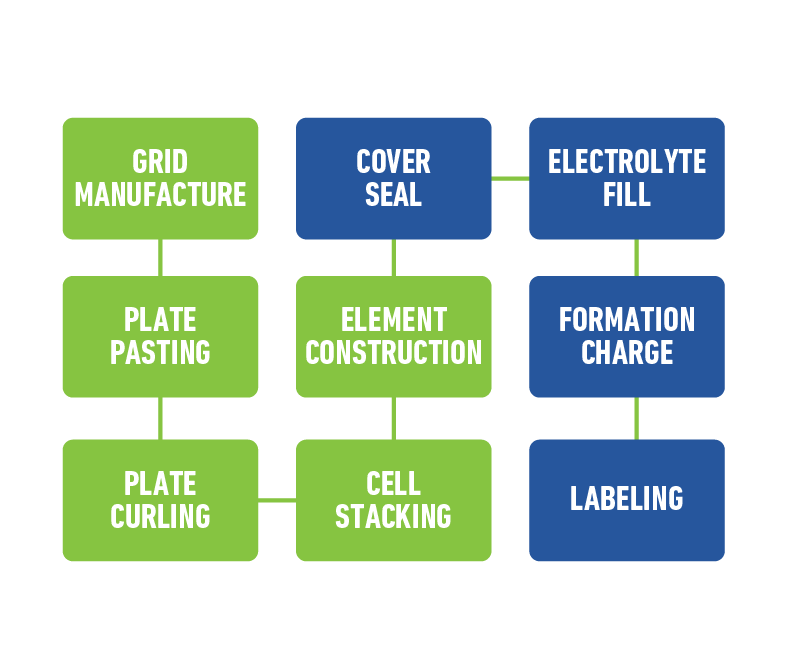
The initial process begins with the manufacturing of grids from an alloy of lead mixed with a small percentage of other metals. The grids conduct the current and provide a structure for the active material to adhere.
Next, a paste mixture of lead oxide – which is powdered lead and other materials – sulfuric acid and water is applied to the grids. Expander material made of powdered sulfates is added to the paste to produce negative plates.
From there, a pasted plate will need to be cured. Curing typically takes place in a controlled environment with warm to high temperatures and modifying humidity for two to four continuous days. During this process, the crystallization growth occurs which binds the paste to the grids. Once cured, the plates are required to completely cool and dry.
Once the plates are ready, they will need to be alternatively stacked with a piece of separator between them. Separators are sheets of porous material that prevent short circuits, yet allow electrical current to flow between the plates. Once appropriately combined, all the positives are connected together, and separately, all the negatives are connected together. This combination of positives, negatives and separators combined is referred to as an element. The elements are then properly oriented and inserted into the battery case and welded together. Elements are typically arranged in series, in order for the two volt cell to reach six, 12 or whatever the intended voltage of the final battery.
Next, a cover is sealed to the top of the case, which contains the connected elements, and the terminal posts are formed outside creating an acid tight seal.
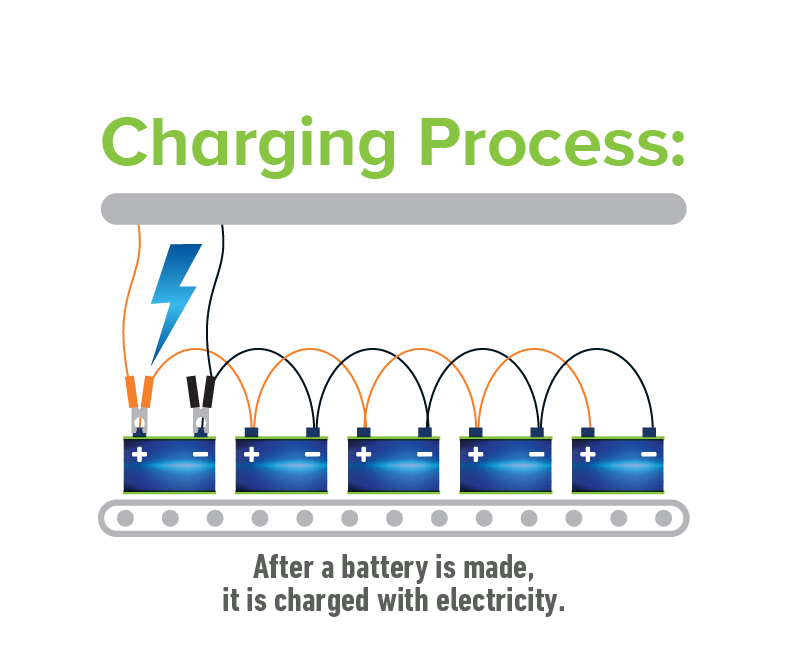
Since the battery construction is now complete, it can be filled with sulfuric acid or electrolyte and placed onto the formation charge. During the formation charge, the battery is connected to an electrical source and charged for many hours. Finally, once a battery is fully formed, it will endure various quality checks and be cleaned and labeled before arriving at a location for sale.
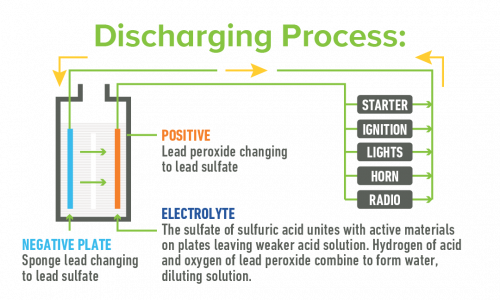
A battery stores electricity for future use. It develops voltage from the chemical reaction produced when two unlike materials, such as the positive and negative plates, are immersed in the electrolyte, a solution of sulfuric acid and water. In a typical lead battery, the voltage is approximately two volts per cell, for a total of 12 volts. Electricity flows from the battery as soon as there is a circuit between the positive and negative terminals. This happens when any load that needs electricity, such as the radio, is connected to the battery.
Lead batteries operate in a constant process of charge and discharge When a battery is connected to a load that needs electricity, such as a starter in a car, current flows from the battery and the battery then begins to discharge. As a battery begins to discharge, the lead plates become more alike, the acid becomes weaker and the voltage drops.
A full charge restores the chemical difference between the plates and leaves the battery ready to deliver its full power. In a vehicle, charging happens when you are driving and the alternator generates current to put back into the battery.
The unique process of discharge and charge in lead batteries means that energy can be discharged and restored repeatedly. This is what is known as the cycling ability in a battery. If the battery won’t start your car, you usually refer to it as dead even though that is not technically correct. A battery that is discharged, from leaving your headlights on or from a damaged alternator, can be recharged at full capacity. Whereas, a battery at the end of its service life can’t be recharged enough to restore to a useful power level, then it is truly dead and must be replaced.
We all believe that advanced lead batteries have the technical capabilities to support next generation energy storage facilities, and we’re already working hard to bring that potential into reality.