About BCI
Battery Council International (BCI) is the leading trade association representing the North American battery industry, an industry...
AC – alternating current
AGM – absorbent (or absorptive) glassmat
Ah – ampere hour
Amp – ampere
BMS – battery management system
CCA – cold cranking amps
CCS – carbon capture and storage
CI – constant current
CSR – corporate social responsibility
CV – constant voltage
DC – direct current
DOD – depth of discharge
EFB – enhanced flooded battery
EOL – end of life
ESG – environmental, social, and governance
EV – electric vehicle
GHGs – greenhouse gases
GWh – gigawatt-hour
HEV – hybrid electric vehicle
MWh – megawatt-hour
OCV – open circuit voltage
PHEV – plug-in hybrid electric vehicle
RC – reserve capacity
REC – renewable energy certificate
SOC – state of charge
SOH – state of health
SRI – socially responsible investing
VRLA – valve regulated lead-acid
ACTIVE MATERIAL — The porous structure of lead compounds that chemically produce and store energy within a lead-acid battery. The active material in the positive plates is lead dioxide and that in the negative is metallic sponge lead.
AFFECTED COMMUNITY — A group living or working in the same area that has been or may be affected by a reporting undertaking’s operation or through its value chain. The local community can range from those living adjacent to the organization’s operations to those living at a distance.
AGM (Absorbent Glass Mat) — A type of non-woven separator material comprised almost entirely of glass microfibers that absorb and retain the electrolyte leaving no free electrolyte in the cell to spill and allow for a recombination of charging gasses. VRLA batteries made with this material are often referred to as “AGM” batteries.
ANODE — The negative electrode. It is the part of a battery that oxidizes and sends electrons to the cathode (the positive electrode) on discharge.
AMPERE (Amp, A) — The unit of measure of the electron flow rate, or current, through a circuit.
AMPERE-HOUR (Amp-Hr, Ah) — A unit of measure for a battery’s electrical storage capacity obtained by multiplying the current in amperes by the time in hours of discharge. (Example: A battery that delivers 5 amperes for 20 hours delivers 5 amperes X 20 hours = 100 Amp-Hr of capacity.)
AMPERE-HOUR THROUGHPUT — The total sum of amp-hour capacity used during cycling over the life of the battery.
AUXILIARY BATTERY — A battery used to power low voltage auxiliary requirements of the vehicle or application.
BATTERY MANAGEMENT SYSTEM (BMS) — An electronic sensing system containing a program that monitors battery condition, performance and health that can be used by the application to make system decisions.
BATTERY STORAGE — The storage of excess energy in batteries for later use, often used in conjunction with renewable energy systems.
BIODIVERSITY — The variability among living organisms from all sources including, inter alia, terrestrial, marine, and other aquatic ecosystems and the ecological complexes of which they are part: this includes diversity within species, between species, and ecosystems.
BIPOLAR BATTERY — A battery which uses a conductive interface to directly connect an anode on one side and the cathode on the other side of an adjacent cell.
BOOST CHARGE — The process of ensuring that the cells and plates within a battery are charged sufficiently for the battery to perform its desired function. Boost charging is typically done for a short duration at a higher-than-normal charge current.
BUSINESS SUSTAINABILITY — The ethical, responsible management of an organization’s continued environmental success related to regulatory compliance, climate disclosures, social impacts, and financial risks.
CAPACITY — The total amount of electrochemical energy a battery can store and deliver to an external circuit. It is normally expressed in terms of Ah or runtime at a desired discharge rate. The nominal or nameplate capacity of a battery is specified as the number of Amp-Hrs or runtime that a conditioned battery should deliver at a specific discharge rate, temperature and cutoff voltage specified by the battery manufacturer. The capacity of a battery is affected by a number of factors such as: active material weight, density of the active material, adhesion of the active material to the grid, number, design and dimensions of plates, plate spacing, design of separators, specific gravity and quantity of available electrolyte, grid alloys, final limiting voltage, discharge rate, temperature, internal and external resistance, age, and life history of the battery. The actual capacity of a battery is not a constant value and is seen to decrease with increasing discharge rate and/or decreasing temperature in addition to age and usage.
CARBON CREDIT — When companies create carbon offsetting initiatives, they receive a transferable or tradeable carbon credit, or token. A credit represents the right to emit greenhouse gases and make up for it elsewhere. A credit represents one ton of carbon dioxide reduced or removed from the atmosphere. In practice, taking advantage of these credits lets owners reduce greenhouse gas emissions to get closer to net zero. The term also refers to purchased credits that will fund emission-reducing projects.
CARBON FINANCE — The financial transactions and mechanisms that support efforts to reduce greenhouse gas emissions or enhance carbon sinks. This can include investments in low-carbon technologies, carbon offset projects, and carbon credits.
CARBON FOOTPRINT — The total amount of greenhouse gases (such as carbon dioxide) produced by human activities, including transportation, energy use, and industrial processes. Reducing carbon footprint is a key component of ESG strategies for companies and investors.
CARBON INTENSITY — Carbon intensity refers to the amount of carbon emissions per unit of energy consumption or economic output. It is used as a measure of the efficiency and sustainability of energy systems and can be used to compare the emissions of different energy sources.
CARBON NEUTRAL — The ideal balance between carbon dioxide emissions produced by human activity and carbon absorption by the atmosphere; the calculation should come to zero.
CARBON OFFSET — A financial instrument that represents a reduction in greenhouse gas emissions and can be traded on carbon markets. Offsets are used to compensate for emissions that cannot be reduced directly and are generated by projects such as renewable energy, energy efficiency, and reforestation.
CARBON PRICING — Carbon pricing refers to the use of economic incentives, such as taxes or cap-and-trade systems, to encourage the reduction of greenhouse gas emissions. By putting a price on carbon emissions, carbon pricing provides an economic incentive for companies and individuals to reduce emissions and invest in low-carbon technologies.
CARBON SEQUESTRATION — Carbon sequestration refers to the process of capturing and storing carbon dioxide emissions, typically from power plants, so that they are not released into the atmosphere. This can be achieved using carbon capture and storage (CCS) technologies, or through the storage of carbon in forests and other land-based sinks.
CARBON TOKEN — A digital asset governed by a smart contract on a blockchain that represents a real-world reduction in one metric ton of carbon dioxide emissions. The asset exists to verify ownership and to simplify the carbon credit trading process.
CATHODE — A positive electrode made of a material that is electrochemically reduced and receives electrons from the anode (the negative electrode) on discharge in an electrochemical reaction that supp-lies electrons (i.e., electricity) to an external circuit.
CONTAINER AND COVER — The reservoir and lid containing the battery parts and electrolyte made from impact and acid-resistant materials.
CELL — The basic electrochemical current-producing unit in a battery, consisting of a positive electrode (set of positive plates), a negative electrode (set of negative plates), electrolyte, separators and casing. It is a single unit housed within one cavity of a monoblock battery container. There are six cells in a 12-volt lead-acid battery.
CHARGE ACCEPTANCE — The ability of a secondary battery to accept energy charge in use or under a test with a given set of charging parameters (temperature, voltage, stand time, battery state of charge).
CELL BALANCING — The process (either manually or through the application of a BMS) of altering individual cell voltage and state of charge through the distribution of charge current to produce uniform cells. This process can help improve the battery’s performance, safety, and longevity.
CHARGE (Q) — A quantity of electrical capacity. The SI unit is the coulomb—equivalent to an ampere-second. However, the ampere-hour is the customary unit for batteries.
CHARGE /CHARGING — The process of applying an external electrical energy source to a battery during which time the electrical energy is converted to stored chemical energy through reactions at the anodes and cathodes of the battery.
CHARGE CURRENT — The rate of energy (flow of electrons) imparted to a battery during charging.
CHARGE CUT-OFF VOLTAGE — The battery voltage at which the charging is terminated when reached under specified charging conditions.
CHILD LABOR — Any work that is likely to be hazardous or to interfere with the child’s education, or to be harmful to the child’s health or physical, mental, spiritual, moral, or social development.
CIRCUIT — An electrical circuit is the path followed by a flow of electrons. A closed circuit is a complete path. An open circuit has a broken, or disconnected, path.
CIRCUIT (Series) — A circuit that has only one path for the flow of current. Batteries arranged in series are connected with negative of the first to positive of the second, negative of the second to positive of the third, etc. A series circuit adds the voltages of each battery without increasing capacity.
CIRCUIT (Parallel) — A circuit that provides more than one path for the flow of current. A parallel arrangement of batteries (usually of like voltages and capacities) has all positive terminals connected to a conductor and all negative terminals connected to another conductor. A parallel circuit adds the capacities of all batteries without increasing battery voltage.
CIRCULAR ECONOMY — The practice of fully keeping products in circulation possible by reducing material consumption, streamlining processes, and collecting waste for reuse. An economic model in which products and materials are designed in such a way that can be reused, remanufactured, recycled, or recovered and thus maintained in the economy for as long as possible (similar to the United Nations definition).
CLEAN TECHNOLOGY —Technologies and processes that are meant to limit negative environmental impact, such as waste and carbon emissions, especially in comparison to fossil fuels. Examples of clean technologies – sometimes referred to as green technologies or eco-technologies – include solar power, wind power, biofuels, recycling, and smart lighting.
CLIMATE ADAPTATION — The act of preparing for and adjusting to climate change’s current and projected consequences (e.g., cities can build seawalls to protect from rising sea levels).
CLIMATE CHANGE — The shifts over time in the average temperature and weather patterns that define specific locations. In particular, climate change has come to mean the rise in global temperatures from heat-trapping gases resulting from mining and using oil, coal and other fossil fuels. Climate change indicators include rising sea levels; increase and severity of extreme weather, such as hurricanes, droughts, and floods; and ice loss at the Earth’s poles.
CLIMATE MITIGATION — The process of decreasing the flow of heat-trapping pollution (e.g., reducing fossil fuel burning by using renewable energy sources may help).
CLIMATE RESILIENCE — The ability to support a community, company, or the natural environment before, during and after a climate event in a timely, efficient manner. The ability of a community, company, or the natural environment to prepare for, recover from and adapt to the impacts of climate change.
CLIMATE RISK —As wildfires, droughts, food scarcity, flooding, hurricanes, and other climate change effects happen, businesses face increased vulnerability. Climate risk describes that vulnerability. It is the potential for climate change to create negative effects on human or ecological systems.
Risks fall into two main categories:
CLOSED LOOP — A circular production process that reuses material waste to create additional products or repurpose recycled materials.
COLD CRANKING PERFORMANCE RATING (CCA) — The rating set by the battery manufacturer indicating the discharge load in amperes which a new fully charged and conditioned battery at -18 oC (0 oF) can continuously deliver for 30 seconds and maintain a terminal voltage equal to or higher than 1.20 volts per cell (7.2 V for a 12 V battery) subject to statistical analysis. European standards (EN) also have a CCA rating defined somewhat differently. Numerically, the values tend to be similar, but they may not always be identical.
CONDUCTANCE (G) — The ability to transmit current in a circuit or battery. It is the reciprocal of resistance and measured in Siemens.
CONSTANT CURRENT/POWER (CHARGE/DISCHARGE) — While charging or discharging the battery, the rate of charge (I) or power (IxV) flowing either into or out of the battery is held constant.
CORPORATE SOCIAL RESPONSIBILITY (CSR) — For-profit companies use the CSR business model to gauge social and environmental benefits alongside organizational goals such as profitability.
CORROSION — The electrochemical reaction between a material, usually a metal, and its environment that produces a deterioration of the material and its properties. The positive lead grids in a battery gradually corrode in service often leading to battery failure. Battery terminals are also subject to corrosion if they are not properly maintained.
CURRENT (I) — The rate of flow of electricity, or the movement of electrons, along a conductor (comparable to the flow of a stream of water). The unit of measure for current is the ampere.
CURRENT (ALTERNATING) (AC) — An electrical current that periodically reverses direction and changes its magnitude continuously with time, in contrast to direct current. A battery does not deliver alternating current.
CURRENT (DIRECT) (DC) — An electrical current flowing in an electrical circuit in one direction only. A secondary battery delivers direct current and must be recharged with direct current in the opposite direction of the discharge.
CYCLE — In a battery, one discharge plus one recharge equals one cycle.
CYCLE LIFE — The count of total cycles for a given standard test profile, measured until the battery is unable to meet minimum test criteria (e.g. discharge voltage or current) Different standardized tests have different sized cycles and testing parameters depending upon the type of battery. Results are not directly comparable.
CYLINDRICAL CELL/BATTERY — A battery cell construction where the positive and negative electrodes and separators are jelly-rolled into a cylindrical shape as opposed to a layered, flat electrode orientation (known as prismatic) and inserted into a tube-shaped housing.
DEEP DISCHARGE — A discharge when a relatively large portion of the battery capacity, for a given rate of discharge, is removed. Typically the voltage cutoff is 1.75 VPC to avoid cell reversal. Deep cycle batteries are designed to be repeatedly deep discharged without significant damage.
DEPTH OF DISCHARGE — The calculated percentage of the amount of capacity removed relative to the full charged capacity available at a given rate of discharge and temperature. The nameplate capacity rating or measured capacity reference value is often used for the full charged capacity.
DIGITAL CARBON FOOTPRINT — The amount of greenhouse gas emissions digital devices, tools and platforms produce. All tech, from cloud computing to mobile phones to internet usage, produces a digital carbon footprint.
DISCHARGING — When a battery is delivering current, it is said to be discharging.
DIVERSITY — A commitment to recognizing and appreciating the variety of characteristics that make individuals unique in an atmosphere that embraces and celebrates individual and collective achievement. Identity is dependent on much more than one dimension of a person’s background. In recognizing and appreciating the many characteristics that make individuals unique in the world, diversity provides solutions to eliminate racial discrimination in the workplace.
DOUBLE MATERIALITY — A concept that requires companies to consider the financial and non-financial impacts of their decisions on people, society, and the environment.
DRAWDOWN — The point at which atmospheric greenhouse gas levels stop climbing and start declining.
ECO-FRIENDLY — A term used to describe products, practices, and technologies that have a minimal impact on the environment and promote sustainability.
ENHANCED FLOODED BATTERY (EFB) — An EFB is a vented (flooded) lead-acid starter battery with additional design features to significantly improve the cycling capability and service life compared to standard flooded batteries, especially for start-stop vehicle applications. Also known as an Advanced Flooded Battery.
ELECTRODE — The combination of active material that electrochemically stores and releases energy and a conducting substrate that supports or contains the material and allows useful electrical energy to flow to an external circuit.
ELECTROLYTE — An ionic (non-metallic) conductor of electricity (typically liquid) placed between the positive and negative electrodes of a battery. Ion movement enables internal current flow. In a lead-acid battery, the electrolyte is sulfuric acid diluted with water that also participates in the chemical reactions.
ELECTRONIC BATTERY TESTER — An electronic device that assesses the condition of a battery through an ohmic measurement such as resistance or conductance, typically without drawing large current loads.
ELECTRONIC WASTE (E-WASTE) — Electronics at or nearing the end of their useful life. Sustainability approaches seek to extend the useful life of devices and use circular economic principles to keep the amount of e-waste to an absolute minimum. The priority is to first reduce waste, then refurbish devices and only then move toward recycling.
ELEMENT — A set of positive and negative plates assembled with separators that makes up one cell.
EMERGENCY PREPAREDNESS PLAN — A plan outlining the steps a building or organization should take in the event of a natural disaster.
EMISSIONS — The release of greenhouse gases (GHGs) and other pollutants into the atmosphere. Emissions can be caused by various human activities, including the burning of fossil fuels, deforestation, and industrial processes.
ENERGY AUDITS — An assessment of a building’s energy usage and potential for improvement, including recommendations for energy efficiency upgrades.
ENERGY EFFICIENCY — The use of less energy to perform the same functions, reducing the amount of energy required to produce goods and services and minimizing greenhouse gas emissions.
ENVIRONMENTAL JUSTICE — Environmental justice aims for fair treatment of all people regardless of race, color, national origin, or income equally regarding environmental laws, regulations, and policies. The approach holds that no group should bear a disproportionate share of negative environmental consequences.
ENVIRONMENTAL, SOCIAL, AND GOVERNANCE (ESG) — Sustainable and ethical interests that can be central to an organization’s financial and corporate interests.
EQUALIZATION CHARGE — The process of ensuring that the cells and electrodes within a battery are all at full charge and that the electrolyte is uniform and free of stratification. This is normally done by charging the battery under controlled conditions (charge current, time and upper voltage limits).
ESG FRAMEWORK — A set of objectives that companies can use to report on ESG issues. The process begins when an organization selects an ESG reporting method.
EV CHARGING — Refers to the charging of electric vehicles, which can be powered by renewable energy sources.
FEED-IN TARIFF — A policy designed to accelerate investments in renewable energy. A policy of this type usually involves long-term government contracts.
FLOAT CHARGE — A constant voltage charging method intended for extended periods of time in which the voltage set point is lowered and optimized for low water loss and reduced grid corrosion while maintaining the battery at a full state of charge.
FLOODED CELL or BATTERY — A cell or battery with electrodes submerged in a free-flowing liquid electrolyte that is not absorbed or otherwise immobilized. Typical designs have venting systems open to the atmosphere for gas escape. They must generally be maintained continuously in a nearly upright orientation to prevent spillage.
FLOW BATTERY — A type of rechargeable electrochemical cell in which chemical energy is provided by two chemical redox components dissolved in liquid electrolytes stored in separate tanks that are pumped through the system on separate sides of a membrane and conductive current collectors. With a simple flow battery it is straightforward to increase the energy storage capacity by increasing the quantity of electrolytes stored in the separate tanks. The electrochemical cells can be electrically connected in series or parallel, so determining the power of the flow battery system.
FORMATION — In battery manufacturing, formation is the process of charging the battery for the first time. Electrochemically, in the presence of sulfuric acid electrolyte, formation changes the electrically inactive lead oxide/sulfate materials in the electrodes into electrically active lead dioxide (positive) and metallic sponge lead (negative).
FUEL CELL — Refers to a type of technology that converts chemical energy into electrical energy, typically using hydrogen and oxygen.
GASSING — The output of hydrogen and oxygen gasses from the respective negative and positive electrodes during the final phase of charging, created from the decomposition of water in the electrolyte.
GEL — Electrolyte that has been immobilized by the addition of a chemical agent, normally fine silica, to prevent spillage and allow gas recombination. Batteries made with gelled electrolyte are often referred to as Gel batteries. Gel batteries are one type of VRLA batteries.
GLOBAL WARMING — Global warming refers to Earth’s heating from trapped greenhouse gases resulting from human activities such as transportation, agriculture, overfishing, fossil fuel energy production and overconsumption.
GREENHOUSE EFFECT — The result of carbon dioxide, methane and nitrous oxides in Earth’s atmosphere trapping the sun’s heat.
GREENHOUSE GAS EMISSIONS — The sum of emissions of various heat-trapping gases. Greenhouse gases include carbon dioxide, methane, nitrous oxides, and fluorinated gases such as hydrofluorocarbons.
GREENHOUSE GAS PROTOCOL — A globally recognized set of reporting and accounting frameworks for managing greenhouse gas emissions from private and public sector operations, value chains and mitigation actions.
GREENWASHING — Deceptive, misleading, or false claims or actions that an organization, product, or service has a positive environmental effect. Whether intentional or unintentional, the practice is detrimental.
GRID — A lead alloy framework that supports the active material of a battery plate and conducts current generated by the active materials to an external connector.
GROUND — The reference zero potential of a circuit. In automotive use, the result of attaching one battery cable to the body or frame of a vehicle that is used as a path for completing a circuit in lieu of a direct wire from a component. Today, over 99% of automotive and LTV applications, use the negative terminal of the battery as the vehicle ground.
HAZARDOUS WASTE — Refers to waste that is potentially dangerous or harmful to human health or the environment, such as chemicals or radioactive materials.
HIGH EMITTERS — A designation given to companies or countries that emit comparatively high volumes of greenhouse gas. Per capita emissions are used to measure the emissions of nations.
HYDROMETER — A device used to measure the concentration of sulfuric acid in the electrolyte through specific gravity of the electrolyte. The specific gravity is often used to estimate the charge level of a battery. A temperature compensation factor is applied to the density measurement to calculate the specific gravity of the electrolyte at a given temperature.
IMPACT INVESTING — An investing strategy that directs money towards companies that create a measurable, positive change in the world. This may also be called socially responsible investment.
IMPACT SOURCING — A sourcing strategy that directs employment and career development opportunities toward people from economically disadvantaged backgrounds.
INTERNAL IMPEDANCE — The opposition to the flow of a small alternating current in battery at a particular frequency combining resistance and reactance. Measured in ohms or milliohms.
INTERNAL RESISTANCE — The opposition to direct discharge current flow within a battery that causes a drop in battery voltage. Measured in ohms or milliohms.
INTERCELL CONNECTORS — Lead structures that connect adjoining battery cells in series, the positive of one cell to the negative of the next.
LEAD SULFATE — The crystallized material that is deposited in each lead-acid battery electrode as a result of discharge of the charged lead materials in the electrodes and sulfuric acid within the electrolyte.
LIFE CYCLE ASSESSMENT — A comprehensive analysis of the environmental impacts of a product, process, or service over its entire life cycle, from raw materials extraction to disposal.
LITHIUM ION BATTERY — A sealed rechargeable battery that uses various cathode and anode materials and lithium ions as the primary ionic conductor in an organic electrolyte.
LOAD TESTER — An instrument that assesses battery performance by drawing a relatively large discharge current from a battery using an electrical load device while measuring voltage.
LOSS AND DAMAGE — Climate-change related consequences that people are unable to adapt to, either because the consequence is too severe or because the affected community doesn’t have access to the resources to adapt. Loss and damage results from sudden natural disasters, such as floods, or gradual change, such as desertification.
LOW WATER LOSS BATTERY — A flooded battery that does not require periodic water addition under normal driving conditions; also referred to as a maintenance-free battery.
MAINTENANCE FREE BATTERY — A battery that normally requires no service watering during its lifetime of use.
MATERIALITY ASSESSMENT — A formal way of assessing stakeholders’ commitment to specific ESG issues and calculates an organization’s ESG score. It works by identifying the impact of a certain issue on a company’s performance and competitiveness in the market.
MEMORY EFFECT – Certain types of batteries, such as nickel-cadmium and nickel-metal hydride, can develop a memory effect when only partially discharged before recharging. This “memory” reduces the capacity of subsequent charges and thus future battery performance. The effect can also be caused by poorly designed chargers.
MITIGATION MEASURES – Refers to the measures taken to reduce the likelihood and impact of natural hazards, such as elevating buildings in flood-prone areas.
MONOBLOCK — A term used to describe a single housing containing individual and connected battery cells.
NEGATIVE — Designating, or pertaining to, a point of electrical potential. The negative battery terminal is the point from which electrons flow during discharge.
NET ZERO — The result of lowering greenhouse gas emissions as close as possible to zero and balancing remaining emissions with removals.
OHM — A unit for measuring electrical resistance or impedance within an electrical circuit or battery.
OHM’S LAW — Expresses the relationship between volts (V) and current (I) in an electrical circuit with resistance (R). It can be expressed as follows: Volts (V) = Amperes (I) x Ohms (R).
At high discharge rates when coupled with the polarized voltage of the battery, the discharge current times the internal battery resistance (I x R) relates to the voltage drop under load within the battery.
OPEN CIRCUIT VOLTAGE — The voltage of a battery measured in the absence of charge or discharge current. After a specified stabilizing rest period it is often used to estimate the charge level of a battery.
OVER-CHARGE/OVER-DISCHARGE — A continued excess charging of a fully charged battery or, conversely, the excess discharging of a fully discharged battery. A lead-acid battery requires more charge than discharge due to inefficiencies, but excessive overcharge is abusive and leads to damage. Over-discharge generally refers continuing discharge passed a cutoff voltage limit and can also lead to damage.
PLATES — Thin, flat structures comprised of a lead conductive grid and active material. The grid supports the active material and conducts electrons out of the cell. Plates are either positive or negative, depending on the active material they hold.
POSITIVE — Designating, or pertaining to, a kind of electrical potential; opposite of negative. A point or terminal on a battery having the higher relative electrical potential. The positive battery terminal is the point to which electrons flow during discharge.
PRIMARY BATTERY — A battery that can store and deliver electrical energy but cannot be recharged. A lead-acid battery is not a primary battery.
PRISMATIC CELL/BATTERY — A cell constructed with flat, rectilinear plates housed in a container with flat interior walls.
PRODUCT RESPONSIBILITY — The responsibility of companies to ensure that their products are safe, ethical, and sustainable, and to minimize any negative impact they may have on the environment or human health.
RAW MATERIALS USAGE — Raw materials usage refers to the extraction, processing, and use of natural resources, such as minerals, fuels, and timber, for the production of goods and services. High levels of raw materials usage can result in environmental degradation, depletion of natural resources, and increased greenhouse gas emissions.
RECHARGEABLE BATTERY — A battery that can have its capacity restored by a charging current.
RECYCLING — The process of collecting and processing waste materials, ideally to make new products. Usually requires treatment, and energy consuming procedure.
REGULATORY COMPLIANCE — A company’s adherence to applicable laws and regulations in its operations. Regulatory compliance is important for ESG as it helps ensure that a company is operating in a responsible and legal manner and that its activities align with stakeholders’ interests.
RENEWABLE ENERGY — Energy sources that are replenished naturally, such as wind, solar, and hydropower. Renewable energy is seen as an important ESG issue because it reduces dependence on finite fossil fuels and helps mitigate climate change.
RENEWABLE ENERGY CERTIFICATE (REC) — Issued when one megawatt-hour (MWh) of electricity is generated and delivered to the electricity from a renewable energy resource.
RESERVE CAPACITY RATING — A rating published by the battery manufacturer that is expressed as the number of minutes to reach 1.75 V/cell volts per cell when a new fully charged battery at 26.7 oC (80°F) is continuously discharged at 25 Amperes, subject to statistical analysis.
RESILIENCY MEASURES — Refers to the measures taken to prepare for and recover from the impacts of natural hazards, such as reinforcing structures or installing backup power systems.
RESISTANCE — The opposition to the free flow of direct current in a circuit or battery. Resistance (measured in units of ohms or milliohms) in batteries is a measure of the rapid change in voltage with respect to the rapid change in current.
RESPONSIBLE INNOVATIONS — Prioritizes ethics and social responsibility in the research, design and production of new technologies or evolutions of existing technology. Responsible innovation posits ethics as a design problem.
REUSE — Using an object again, without treatment.
RISK MANAGEMENT — The processes and practices a company uses to identify, assess, and manage potential risks to its business, such as environmental, social, or financial risks. Effective risk management is important for ESG as it helps ensure that a company is prepared to address potential risks and minimize their impact on its operations and stakeholders.
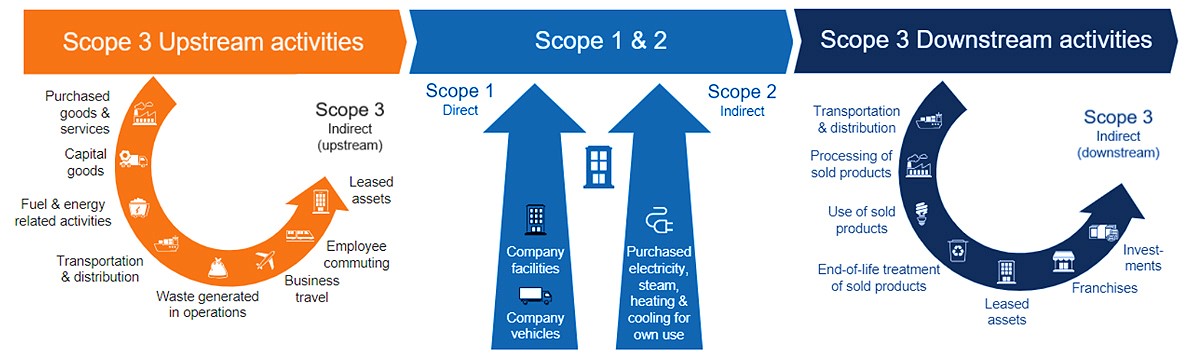
SCOPE 1, 2, 3 EMISSIONS — Developed by the Greenhouse Gas Protocol, scopes give organizations a way to categorize their emissions. Organizations may find it easier to control scopes 1 and 2, but scope 3 emissions are the most difficult to track.
SEALED BATTERY — A battery that does not have accessible vent caps or a VRLA (valve regulated lead-acid battery).
SECONDARY BATTERY — A battery that can deliver electrical energy and can be recharged by passing direct current in a direction opposite to that of discharge. A lead-acid battery is a secondary battery.
SELF-DISCHARGE — Internal chemical reactions taking place within the electrodes that result in a loss in stored charge.
SEPARATOR — A porous membrane divider between the positive and negative plates in a cell that allows the flow of ionic current to pass through it, but not electronic current. Separators are made from numerous porous materials impervious to electrolyte and oxidation such as: polyethylene-silica, glass fiber, porous rubber, PVC, phenolic cellulose, etc.
SERVICE LIFE — The normal operating period of time for a battery in an application until it no longer satisfies the minimum application requirements.
SHORT CIRCUIT — An unintended current bypass in an electrical device or wiring. Outside a battery a short circuit is established when an unintended conductive path is established between the two terminals of a battery. Inside a battery, a cell short circuit is the result of contact between the positive and negative plates that will cause a cell to discharge and render the battery useless.
SOCIALLY RESPONSIBLE INVESTING (SRI) — An investment approach that considers both financial returns and social and environmental impact. Investors who follow SRI principles seek to invest in companies that have positive ESG practices and avoid companies that have negative ESG practices.
SODIUM ION BATTERY — A sealed rechargeable battery similar to Lithium Ion that uses sodium ions (instead of lithium) as charge carriers.
SOLAR — Refers to energy derived from the sun, typically using photovoltaic panels.
SPECIFIC GRAVITY (Sp. Gr. or ‘SG’) — Specific Gravity is a measure of the sulfuric acid electrolyte concentration in a battery at a specific temperature. This measurement is based on the density of the electrolyte compared to the density of water and is typically determined by the use of a hydrometer (see Hydrometer). By definition, the specific gravity of water is 1.00 and the specific gravity of the sulfuric acid electrolyte in a typical fully charged lead-acid battery is 1.265-1.300. Specific gravity measurements are typically used to determine battery charge level or if the battery has a bad cell.
STAKEHOLDER ENGAGEMENT — Refers to a company’s communication and interaction with its stakeholders, including shareholders, employees, customers, and local communities, to understand and address their concerns and expectations. Stakeholder engagement is important for ESG as it helps companies identify potential ESG risks and opportunities and ensure that their activities align with the values and interests of their stakeholders.
STATE OF CHARGE — The percentage of remaining discharge capacity or discharge time available in a battery under prevailing conditions of discharge compared to a fully charged battery under the same or defined conditions: SOC = 100% x Cremaining / Ccharged
Because of various methods of defining state of charge, it is problematic to use without setting specific calculations.
STORMWATER — Refers to water that falls as precipitation and flows over land and impervious surfaces, rather than infiltrating into the ground.
STRATIFICATION — The unequal concentration of electrolyte due to density gradients from the bottom to the top of a cell. This condition is encountered most often in batteries recharged from a deep discharge at constant voltage without a great deal of gassing for mixing wherein recharged higher concentration sulfuric acid sinks to the bottom of the cell. Continued deep cycling of a ‘stratified’ battery will result in softening of the bottoms of the positive plates and sulfation of the bottoms of the negative plates. Equalization charging is a way to avoid acid electrolyte stratification.
STRUCTURAL RISKS — Refers to the potential dangers posed by the physical structure of a building, such as structural failures or collapse.
SULFATION — The generation or conversion of the normal lead sulfate discharge crystals in the plates to a state that resists recharge. Sulfation often develops when a battery is stored or cycled in a partially discharged state at warm temperatures. It is a vague term for low charge acceptance in a discharged condition with no quantitative measurement.
SUPPLY CHAIN MANAGMENT — A company’s practices and policies related to managing its suppliers and ensuring they meet ethical and environmental standards.
SUPPLY CHAIN TRACEIBILITY — In sustainability, traceability not only identifies, tracks, and traces materials and commodities, but it also verifies sustainability claims across the value chain.
SUSTAINBLE DEVELOPMENT — A development path that meets the needs of the present generation without compromising the ability of future generations to meet their own needs.
SUSTAINABILITY — The ability to meet present needs without compromising the needs of future generations. In practice, sustainability aligns environmental protection, human well-being, and economic development.
TERMINALS — The electrical structures on the battery to which the external circuit is connected. Typically, batteries have either top-terminals (posts) or side-terminals. Some batteries have both types of terminals (dual-terminal).
THERMAL RUNAWAY — An uncontrolled increase in battery internal temperature during a voltage-controlled charging process where the current rate increases as the battery temperature continues to rise causing the electrolyte to boil away.
TRANSPARENCY — A company’s openness and accessibility in sharing information about its operations, performance, and governance. Transparency is important for ESG as it helps ensure that a company is accountable and responsible in its activities and that stakeholders have access to information to make informed decisions.
TRICKLE CHARGE —A technique used to describe maintaining the charge level of a battery by charging with a small current either through float charging (which is constant voltage) or a small constant current charge.
VALVE REGULATED LEAD-ACID BATTERY (VRLA BATTERY) — A battery constructed with a fully enclosed case venting system sealed with a 1-way valve, under pressure above atmospheric, where venting of gasses is regulated through the valve that operates in a normally closed position. This configuration enables an oxygen charge shuttle reaction (recombination) inside the battery. When oxygen produced at the positive electrode reaches the negative electrode, the negative electrode is discharged slightly removing the oxygen and limiting the production of hydrogen gas. Instead the VRLA battery regenerates water and no excessive gasses for venting. The valve ensures that air (extra oxygen) never enters the battery to discharge the negative electrode. If the internal pressure exceeds the valve pressure limit by a fixed amount, the battery will vent to limit further pressure rise. Gel and AGM are two types of valve-regulated batteries.
VENTS — Mechanisms that allow gasses to escape from the battery while retaining the electrolyte within the case. Flame arresting vents typically contain porous disks that reduce the probability of an internal explosion as a result of an external spark. Vents come in both permanently fixed and removable designs.
VENTED BATTERY — A battery where the internal cell components (electrodes and electrolyte) are subject to normal atmospheric pressure, and gasses produced are allowed to pass through an engineered venting system.
VOLT — The unit of measure for electrical potential.
VOLTAGE — An electromotive force or potential difference expressed in volts.
VOLTMETER — An electronic device used to measure voltage, normally in a digital format.
VOLTAGE DROP — The net difference in the electrical potential (voltage) when measured across a resistance or impedance (ohms) with applied current. See Ohm’s Law.
VRLA — See Valve Regulated Lead-Acid battery.
WASTE MANAGMENT — The collection, transport, treatment, and disposal of waste, with a focus on reducing the negative impact of waste on the environment and human health.
WATER MANAGEMENT — The process of managing water resources in a sustainable manner, including ensuring access to clean drinking water, reducing water waste, and protecting water ecosystems.
WATT — The unit for measuring electrical power, i.e., the rate of doing work, in moving electrons by, or against, an electrical potential. Formula: Watts = Amperes x Volts.
WATT-HOUR (Watt-Hr, Wh) — The unit of measure for electrical energy equivalent to a power consumption of one watt per for one hour.
ZERO WASTE — The concept of managing products, packaging, and materials responsibility to minimize environmental harm.
I was fascinated by the many facets of lead batteries that I never knew existed…