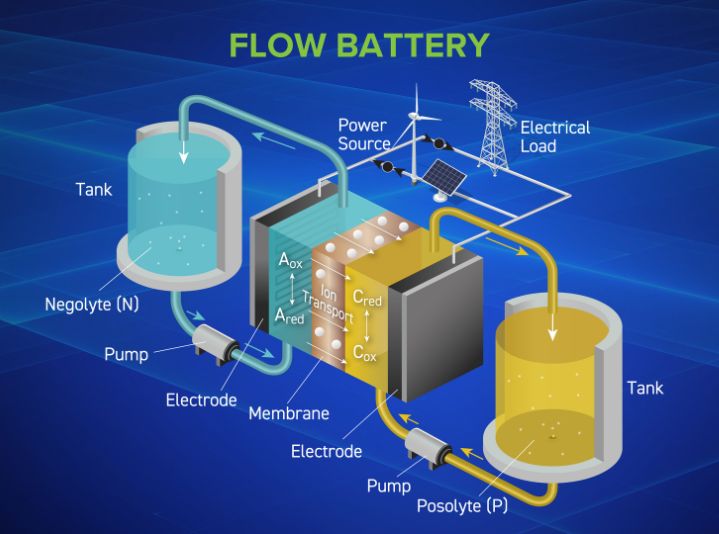
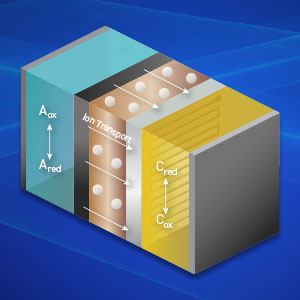
Flow batteries are notable for their scalability and long-duration energy storage capabilities, making them ideal for stationary applications that demand consistent and reliable power. Their unique design, which separates energy storage from power generation, provides flexibility and durability. Ongoing advancements are enhancing their efficiency, cost-effectiveness, and environmental sustainability
Renewable Energy Storage
The need to harness and utilize renewable energy has never been greater. Lead batteries can provide energy storage capabilities for...