Thank you to the researchers that submitted a poster for the 2025 BCI Battery Poster Research Showcase!
2025 Winners & Submissions
Thank you to the researchers that submitted a poster for the 2025 BCI Battery Poster Research Showcase!

Local variation in electrode and electrolyte composition contributes to large-scale changes in overall utilization and cycle life of lead acid batteries. Here, we outline the potential for ultrasonic characterization of lead acid batteries to diagnose local changes in state of charge (SOC) and state of health (SOH). Using 2V cells constructed with commercial battery plates, we show that the amplitude and time-of-flight of ultrasonic signals is highly sensitive to changes in the bulk modulus and density of lead battery materials and acid specific gravity during cycling. Using high-rate partial state of charge cycling, we also show that these changes can be used to track the onset of sulfation on the negative electrode. This nondestructive, analytical tool can provide crucial feedback for emerging battery management systems and could potentially be used for quality control measures during manufacturing.

Redox flow batteries (RFBs) are a promising technology for long duration energy storage (LDES) because of their theoretically long cycle life and scalability. Although the state-of-the-art RFB is vanadium-based, it is not economical due to vanadium’s price volatility and low voltage. Thus, RFBs with alternative electrolytes must be explored. Organic RFBs are attractive due to their low-cost, earth-abundant materials, however, many systems suffer from poor stability and low energy density. Given the vast parameter space for RFB electrolytes, including active materials and supporting electrolytes, the pace of research urgently needs to be quickened to identify systems that are stable and have high energy densities. To accelerate the development of RFBs, we have built an automated flow battery cycler station that can explore a large parameter space and leverage artificial intelligence (AI). The automated platform contains sixteen electrochemical testing channels, each comprised of four peristaltic pumping channels and four selector valves that deliver electrolyte to the flow cell. One software controls both the electrolyte delivery system and the electrochemical testing, resulting in complete automation and closed-loop capabilities. To test the automated station, we collected electrochemistry results for commonly proposed redox active materials like hydroxy-TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxy) and supporting salts like lithium bis(trifluoromethanesulfonyl) imide (LiTFSI). The automated system was used to explore a wide range of supporting salt concentrations without human intervention, underscoring the utility of the cycler station and its potential for use in an autonomous discovery platform to accelerate LDES technology development even further when coupled with machine learning optimization algorithms.

Lead acid batteries (LABs) are widely used in SLI (Starting, Lighting, and Ignition) and stationary applications due to their low cost, high recyclability, and robust safety. However, their operational challenges—low specific capacity, limited cycling life, and active material degradation—continue to constrain long-term performance. In this study, we employed synchrotron X-ray microtomography and energy dispersive diffraction (EDD) to investigate the chemo-mechanical evolution of LAB cells during formation and cycling. Our custom-designed cells mimic actual LAB structures while enabling operando analyses. The observed decrease in utilization after each cycle is consistent with both electrochemical tests and EDD patterns, and this degradation is further confirmed by tomographic imaging. The positive and negative electrodes undergo distinct active materials conversion and gas evolution, resulting in different void evolution behaviors. Additionally, the positive electrode’s current collector develops a corrosion layer, whereas the negative electrode exhibits a volume increase. These findings underscore the complex interplay of active materials conversion, gas evolution, and electrode morphology in determining LAB performance, offering new insights into degradation mechanisms and informing strategies for improved LAB design.

Traditionally, lead acid batteries utilize lignosulphonates as additives called expanders to improve performance and limit degradation from passivation and other structural changes. While they promote better discharge performance, they do slow down charge acceptance – via a mechanism that is not entirely understood. This ambiguity is due to the complexity of the molecules and uncertainty about exact chemical identity. To better provide guidance on the design of these additives, we have developed model expander molecules (MEMs). These lignin-inspired molecules are much simpler in complexity, allowing them to be characterized in more detail (e.g., molecular weight, functionality). In addition to these more holistic properties, electronic properties were calculated utilizing density functional theory (DFT), providing a greater understanding of what properties govern enhancements in discharge and charge. In this work, MEMs were evaluated using a rotating disk electrode setup with flat lead in sulfuric acid in the presence of MEMs, giving us the ability to determine the MEMs enhancement. Combining electrochemical data with the calculated chemical properties, we begin establishing a framework to 1) understand what properties impact charge and discharge characteristics for more thorough design rules and 2) predict novel compounds with targeted molecular features towards finding better additives. This is accomplished using predictive machine learning algorithms to calculate charge and discharge characteristics. In its current state, a variety of MEMs have been discovered that can enhance both charge and discharge capacity, and their properties are being scrutinized for further chemical discovery and the design of advanced expanders for lead acid batteries.

The battery pack for Longhorn Racing Solar’s single-occupant solar vehicle is engineered to compete in two demanding events: the Formula Sun Grand Prix (FSGP) and the American Solar Challenge (ASC). FSGP’s race-style format stresses the battery with rapid accelerations and decelerations, while ASC’s 1500-2000 mile endurance race on real roads requires robust design and sustained performance over extended runtimes. Efficiency, safety, and energy density were prioritized under strict size and weight constraints. Efficiency was achieved by minimizing internal resistance losses and optimizing weight to enhance performance and reduce energy consumption. Positioned directly behind the driver, the battery incorporates a custom-designed Battery Protection System (BPS), which replaces off-the-shelf solutions used by most teams. This system allows exceptional customizability and seamless integration with the pack, enabling advanced monitoring of individual module temperatures and voltages. The BPS isolates the battery during anomalies, triggers a fault state, and alerts the driver, ensuring optimal safety and reliability. The pack features a 32S 9P configuration, delivering a nominal voltage of 118V, a maximum current output of 70A, and a capacity of 5.22 kWh in a compact form factor. The enclosure, made of carbon fiber, Kevlar, and Gurit Kerdyn foam core, provides insulation, fire resistance, and structural integrity while maintaining a lightweight design. Forced-air cooling ensures thermal stability, with liquid cooling solutions under consideration for further optimization. Modular and serviceable, the pack uses linear latches with a cable release system for secure mounting and quick removal. This innovative system meets the rigorous demands of solar vehicle competitions.

Organic additives are essential for optimizing the performance and longevity of lead-acid batteries, as they enhance electrochemical properties and overall efficiency. By increasing the surface area of active materials, additives facilitate electrochemical reactions, thereby boosting the battery’s discharge capacity. Understanding the structure-activity relationships of these additives is crucial for tailoring their properties to enhance battery performance. Systematic studies using cyclic voltammetry evaluated the electrochemical characteristics of over a hundred model expander molecules (MEMs), providing insights into their ability to improve or decrease the discharge and charge processes of the negative active material. Based on this workflow, an autonomous system can exponentially accelerate the screening of MEMs by automating data collection and analysis, significantly increasing throughput and precision. To accelerate the screening process, we have implemented the Electrochemical System for Molecule Autonomous and Rapid Testing (ESMART). This system is designed to allow for mixing up to six simultaneous solutions containing different MEMs or electrolyte compositions. Our platform is controlled using a Python-based interface, allowing for the automation of electrochemical experiments and data analysis. This approach enables the rapid processing of extensive datasets and serves as a key pillar in predicting performance metrics such as discharge enhancement factors (DEF) and charge enhancement factors (CEF). This advancement supports the establishment of design principles for expander molecules tailored for advanced lead-acid battery applications. Furthermore, the ability to quickly and accurately assess a wide range of MEMs not only enhances the understanding of additive behavior but also accelerates the innovation cycle, paving the way for next-generation energy storage solutions for any application.

With the development in science and technology and increased demands of energy, lead acid batteries have attracted the attention of researchers because of their safety, simple manufacturing, low cost, and 99+% recyclability. However, certain issues like sulfation limit the life cycle of the batteries. Sulfation is the irreversible formation of lead sulfate crystals on the battery’s negative electrode, which is related to the size and shape of the crystals. Various strategies have been employed to overcome this issue. Expanders are added to the negative electrode, which affect the morphology of lead sulfate crystals and prevent the loss of performance of the negative electrode. These include lignosulfonate, carbon black, and barium sulfate. Lignosulfonates are complex molecules with many different functional groups, which improve the discharging capacity but impede charging. It is still unclear which functional groups of lignosulfonates are responsible for improved performance and which have detrimental effects. It would be ideal to develop expander molecules that will improve both charge and discharge capacity. To design such molecules, it is important to understand the atomic level interactions between lead species and lignosulfonates. Small molecules with functional groups that mimic the functional groups of lignosulfonates were synthesized and named Model Expander Molecules. These molecules were synthesized by sulfonation and alkylation of simple organic starting materials and characterized by NMR spectroscopy and ESI mass spectroscopy. When applicable, the absence of inorganic byproducts was confirmed by powder diffraction and energy dispersive X-ray spectroscopy. Electrochemical studies were conducted in 5 M sulfuric acid solution and stability was tested by cyclic voltammetry. For further electrochemical studies, these molecules were sent to Argonne National Lab before testing in a real device.

Lead Acid Battery (LAB) is a reliable energy storage solution for vehicles and future electrical grids, presenting low cost, high power, and highest recyclability rates than any other battery technology. However, low material utilization, limited cycle life, and, most importantly, slow rechargeability are key technical limitations that need to be addressed for advanced lead battery systems. At the molecular levels, the process of converting PbSO4 back into Pb at the negative electrode or PbO2 at the positive electrode involves a combination of chemical and electrochemical steps, which need to be better understood to afford control over the recharging rates and prevent cycle life issues such as sulfation. To gain insights into the charging process of PbSO4, in this work we use a double injection precipitation method to synthesize lead sulfate particles with controlled size and shape. By tuning the synthesis conditions, we were able to synthesize particles with different size ranges: 150-170 nm, 250-270 nm, 300-340 nm, and 1.2 µm, in two different shapes, rhombohedral and cubic. These chemically synthesized lead sulfates were used as platform to understand the effect of size and shape in the charging step on both negative and positive electrodes. For both particle shapes, smaller particles charge faster than bigger particles. However, for similar sizes, rhombohedral particles are far more efficient in charging than cubic-shaped particles, and thus, rhombohedral PbSO4 presents the highest kinetic charge acceptance. This study offers new insights on how to control the charging process of lead batteries, with possible consequences to its manufacturing and improving the formation step, reducing the energy use in manufacturing and towards the development of advanced lead acid batteries.

Principal to motor vehicles, power retention systems, and many other applications, rechargeable lead acid batteries facilitate the need for power storage due to their innate reliability and safety. The primary reaction that occurs within these batteries creates insoluble lead sulfate at both electrodes. This accumulation causes gradual impedance to battery performance and eventual inoperability. A common method used by manufacturers to combat this effect is to include additives known as expanders, which improve performance and cycle life by influencing lead sulfate formation. Lignosulfonates are one expander that can be used to significantly increase battery charging performance at the cost of discharge interference. The lignosulfonate expanders affect morphology of the lead sulfate and the porosity retention of the negative electrode, but the atomic level interactions and mechanisms are not known. Our group has proposed to gain insights into why this happens by synthesizing model expander molecules (MEMs) that mimic specific functional groups of lignosulfonates. The MEMs’ effects on charge and discharge are then analyzed utilizing experimental conditions akin to the environment of a lead acid battery. For this project, lead sulfate is precipitated out by controlled additions of lead nitrate to an aqueous solution of sodium sulfate and a chosen MEM. The lead sulfate samples are dried and then analyzed with powder x-ray diffraction and scanning electron microscopy (SEM). The impact of MEM and addition rate is qualified by visual inspection of particle morphologies in SEM images. Furthermore, to gain quantitative insight, a novel technique utilizing Meta’s Segment Anything Model is applied to SEM images to identify and isolate particles to subsequently determine approximate exposed surface areas and distributions. These results are then correlated to the MEMs effects on discharge capacity and kinetic charge acceptance.

Lead acid batteries provide a day-to-day reliable energy storage application in various fields like automotive, standby power, renewable energy, telecommunication, industrial and robotics.1 In addition to the electrochemically active lead species, these batteries contain several additives that improve performance and cycle life. Lignosulfonates (LS) are organic biopolymers that are used as additives in a variety of applications, including the production of lead acid batteries2 (Figure 1). In lead acid batteries, LS serve as organic expanders to improve the performance of the battery’s storage capacity and extending its service life, as well as by acting as a wetting agent and improving the conductivity of the electrolyte, which tends to improve battery efficiency.3 To be able to understand the interaction of specific functional groups with lead species in detail, small molecules that mimic portions of LS can be used as model expander molecules (MEMs). In this study, we designed and synthesized lignosulfonate-based MEMs (Figure 2). The MEMs stability under conditions relevant for battery applications was investigated by cyclic voltammetry in 5 M H2SO4. Additionally, the interaction of the MEMs with Pb2+ and their stability at elevated temperature and in 5 M sulfuric acid was studied by spectroscopic, diffraction and single-crystal X-ray diffraction techniques.

Batteries are poised to play a key role in a sustainable energy future as they are essential for scalable energy storage solutions, particularly for intermittent renewable sources such as solar and wind. Battery condition indicators, such as the state of charge (SOC) and state of health (SOH), are critical for effective battery monitoring and control. However, these indicators are not easily accessible from simple voltage/current measurements and must instead be estimated using models. This is challenging because they are influenced by operating conditions such as temperature and charge/discharge rates, resting periods, etc. Electrochemical impedance spectroscopy (EIS) is a very useful tool that could be used to track batteries’ SOH because of its accurate determination of equivalent resistances that help us characterize electrochemical processes via the ohmic (solution) resistance (Rs), charge transfer resistance (Rct), and double layer capacitance (Cdl) that in turn can help us estimate electrochemical active surface area (ECSA). In this work, we used EIS to track Cdl, Rct, and Rs of the positive active materials (PAM) of lead acid batteries to probe battery failure mechanisms such as materials loss and grid corrosion that are influenced by the charge cut-off potential and the presence of gassing reactions. We observed a correlation between Rct and Rs because gassing causes material loss due to adhesion and cohesion changes within the active material (expansion) and grid corrosion. A direct correlation was also observed as discharge voltage drop seems linked to a decrease in Cdl, ECSA and electrode capacity. Thus, EIS obtained metrics such as Cdl, Rct and Rs could serve as state of health indicators for positive active materials of lead acid batteries.

Despite lead-acid batteries widespread use as an energy storage solution due to their abundant and low-cost materials, improving their durability requires a deeper understanding of negative active material (NAM) processes affecting cycle life. Our group previously demonstrated how discharge rates influence PbSO₄ particle size/layer thickness, governing NAM capacity and battery performance. While those studies were performed on well-defined lead surfaces, establishing the connection to the performance in high surface area pasted electrodes is paramount to connect the fundamental processes for the design of high material utilization and long-lasting electrodes. This work focuses on using pasted NAM electrodes to investigate the impact of discharge rates, depth of discharge (DOD), and overcharge on performance, its degradation and material evolution. Through electrochemical impedance spectroscopy the electrochemical surface area (ECSA), high-frequency resistance, and charge transfer resistance, were determined to establish a connection to Peukert’s law. Discharge capacities normalized by ECSA aligned the expected Peukert curve from flat Pb surface. However, the 3D porous structure, higher discharge rates and deeper DODs lead to inconsistent capacity retention and reduced cycle life. Even at lower DODs, prolonged overvoltage conditions at gas evolution potential induced loss in capacity retention, likely due to active material disturbance by gas bubbles. Comparisons of throughput capacities indicate that slower cycling rates and lower DOD conditions yield higher material utilization and extended cycle life. These findings provide valuable insights into how specific failure mechanisms contribute to performance degradation, establishing groundwork to create mitigation strategies and enhance the longevity and efficiency of lead-acid batteries.

The thermal and chemical degradation of battery active materials is a critical area of study for improving safety and thermal stability in energy storage systems. The research explores the mechanisms behind thermal runaway, quantifies heat generation, investigates reaction kinetics, and analyzes degradation pathways through a combination of experimental and modeling approaches. Advanced calorimetry techniques, including Differential Scanning Calorimetry (DSC), Thermogravimetric Analysis (TGA), and Accelerating Rate Calorimetry (ARC), are employed to examine material behavior under varying thermal conditions. To achieve a comprehensive characterization, evolved gas analysis (EGA) and X-ray diffraction (XRD) are used in parallel to identify gas evolution pathways and structural transformations during degradation. These techniques provide critical insights into the physicochemical changes occurring in battery materials at elevated temperatures Moreover, the modelling framework focuses on estimating heat generation, deriving kinetic parameters, and simulating thermal runaway scenarios at the cell level. This integrative approach bridges experimental observations with theoretical models, enabling a deeper understanding of thermal events in batteries. By combining state-of-the-art experimental techniques with robust modeling, this research contributes to the broader goal of mitigating safety risks in modern battery technologies.

Expander molecules like Vanisperse A are added to the negative electrode pastes used in lead acid (PbA) batteries to promote high surface area and favorable discharge performance. Despite these advantages, expander molecules typically inhibit charging rates, limiting the use of PbAs in advanced applications that require repeated deep discharge/charge cycling. A deeper understanding of the atomic-level mechanisms that control additive-lead species interactions is necessary to optimize expander molecules for both discharge and charge performance. A collaborative project between government, academia, and industry has screened over one hundred model expander molecules (MEMs) using cyclic voltammetry, density functional theory, and various spectroscopy methods to characterize their chemical and electrochemical stability and performance properties. The MEMs are categorized by their lignin structural motifs and the presence of functional groups, e.g., sulfate, sulfonate, and carboxylate, with the goal of establishing structure-function relationships. Discharge (DEF) and charge (CEF) enhancement factors were established as the metrics for electrochemical performance relative to sulfuric acid without expanders as the baseline. Four categories of expander molecules were defined based on their DEF and CEF values: traditional, e.g., Van A, inhibitors, enhancers, and rheology modifiers. The set of materials evaluated to date demonstrates that expander molecules that can enhance both the discharge and charge performance are possible and do exist. On the other hand, the inhibitor class may lead to a deeper understanding of the expander degradation processes and their impact on cycle life. These results help us identify the design rules for expander molecules targeted to advanced PbA applications.

Lead acid batteries provide a day-to-day reliable energy storage application in various fields like automotive, standby power, renewable energy, telecommunication, industrial and robotics. 1 In addition to the electrochemically active lead species, these batteries contain a number of additives that improve performance and cycle life. Lignosulfonates (LS) are organic biopolymers that are used as additives in a variety of applications, including the production of lead acid batteries (Figure 1) 2. In lead acid batteries, LS serve as organic expanders to improve the performance of the battery’s storage capacity and extending its service life, as well as by acting as a wetting agents and improving the conductivity of the electrolyte, which tends to improve battery efficiency. 3 To be able to understand the interaction of specific functional groups with lead species in detail, small molecules that mimic portions of LS can be used as model expander molecules (MEMs). In this study, we are designing and synthesizing lignosulfonate-based MEMs. A series of MEMs were prepared from different synthetic methods (Scheme 1). The MEMs’ stability under conditions relevant for battery applications was investigated by cyclic voltammetry in 5 M H2SO4. Additionally, the interaction of the MEMs with Pb2+ and their stability at elevated temperature and in 5 M sulfuric acid was studied by spectroscopic and diffraction techniques.

The irreversible transition to clean renewable energy requires the deployment of energy storage systems at unprecedented scales. Lead batteries have the potential to continue serving as a crucial energy storage technology for a sustainable decarbonized energy economy because it is earth-abundant, inexpensive, and 99% recyclable. To advance the design of lead batteries with higher material utilization, fast recharge rates, and long-cycle life, will require a fundamental understanding of the electrochemical and chemical processes happening at the atomic and molecular scales. Here we discuss how the use of well-defined lead electrodes allows us to gain insights into the fundamental limits of discharge capacity and recharge rates. We unveil the relationships between discharge rates and PbSO4 particle size/layer thickness that ultimately governs the maximum discharge capacity accessible as a function of discharge rate on both the negative and positive lead electrodes. This data helped us develop a mathematical model that captures the key processes concerning lead ion and (bi)sulfate ion gradients coupled to nucleation and growth that explain, from first principles, the origin of the well-known empirical Peukert law. In this context, we explored how variables such as acid concentration, temperature, and the presence of lignosulfonate additives in the electrolyte further influence discharge capacity, allowing us to understand how thermodynamic, kinetic, and mass transport play a huge role in controlling the accessible capacity of the electrodes. This study paves the way for an in-depth understanding of the important variables in designing a lead acid battery performing at its full potential.

The malleable nature of lead oxides ranging from PbO to PbO2 plays a significant role in the positive electrode of lead batteries. Drawing from decades of thermal studies, engineering a conductive PbO1+x phase in the corrosion layer is necessary to avoid premature capacity loss driven by stoichiometric PbO. Within the active material, both α- and β-PbO2 are thought to be nonstoichiometric, with both oxygen [2] and Pb vacancies that are accompanied by proton interstitials[1]. The importance of non-stoichiometric retention was found in a neutron study which monitored the lead site’s vacancies in the PAM for conventional and fast charged batteries and showed a decrease from βPbO2 stoichiometry through the first 250 cycles with the fast charged battery continuing 750 cycles further than the conventional battery[3]. Our work sets out to provide a better understanding of non-stoichiometric phases and the conditions where they form to improve battery life and performance. We revisited PbOx phases associated within the corrosion layer using in situ diffraction and x-ray absorption and found the presence of metastable Pb3O5 and Pb2O3 phases during thermal decomposition of β-PbO2. Separately, changes in the stoichiometry of β-PbO2 in an electrochemical environment were also investigated using in situ diffraction during cycling of a Planté cell at oxidizing conditions. Both studies were supported by density functional theory calculations which provide context on the overall stability and structure of these phases and the origins of their electronic and oxygen mobility.

The lead-acid battery is a time-tested and robust rechargeable battery technology that has withstood the test of time and is still widely applicable owing to its reliability and high power-to-weight ratio. However, its integration into grid-scale stationary energy storage applications faces challenges, as its performance lags newer battery chemistries. The charge-discharge cycles in lead-acid batteries involve multiscale chemical, morphological, structural, and microstructural evolution, driven by repeated dissolution-nucleation-precipitation of active materials under an applied electric field. Understanding these interfacial-driven processes at the atomistic and molecular scales, as well as elucidating the role of additives facilitating these multiscale transformations, are crucial for enhancing battery life and performance. At PNNL, we have adopted a multimodal analysis approach to uncover the underlying mechanisms occurring at the electrode-electrolyte interfaces in Pb-acid batteries. By utilizing a combination of in-situ and ex-situ tools, we address the most challenging issues surrounding the dissolution-nucleation and phase transformation regimes of active materials. This presentation will discuss our efforts to provide atomistic insights into PbOx phase evolution during in-situ heating, PbSO4 nucleation over native and strontium-doped barite surfaces, and the ion dynamics of sulfuric acid-based electrolytes, all derived from our multimodal analysis. Our goal with these multimodal analyses is to establish a predictive regime for designing active materials with outcomes that can withstand the harsh demands of grid-scale stationary energy storage applications.

Lead acid batteries represent the oldest rechargeable battery technology, still widely used. In addition to automotive applications, exploring their application for large-scale energy storage is desirable. However, the sulfation process in the battery is affecting its charging capacity and performance. Lignosulfonates, which are also referred to as “negative expanders”, are used as an additive in lead acid batteries to overcome some of the issues related to sulfation. Negative expanders perform versatile functions in batteries. They affect the morphology of lead sulfate crystals, the rate of oxidation of lead sulfate, and reduce the sintering of the porous active mass. Lignosulfonates are complex molecules with multiple functional groups, but which functional group interacts with the battery component and improves the performance is still unknown. So, model expander molecules that resemble the various groups of these commercial expanders were synthesized instead. The main motivation of this project was to analyze these interactions and how these interactions can be exploited to enhance the performance of lead batteries. In this research, the synthesis of various MEMs was accomplished by alkylation and sulfonation reactions. The purity of the MEMs was characterized by proton nuclear magnetic resonance (H-NMR) spectroscopy and elemental analysis. Moreover, electrochemical properties were investigated by Cyclic Voltammetry. Their effect on the charge/discharge behavior of lead electrodes in 5 M sulfuric acid was studied by collaborators at Argonne National Laboratory, and several compounds were found to improve charge, discharge, or both.

Lead-acid (Pb-acid) battery cells are a growing electrochemical choice for renewable energy grid-storage, while remaining the premier choice for combustion engine start-up sources. Up until recently, very few non-destructive experimentation techniques have been utilized for Pb-acid cell characterization – such techniques are useful to identify performance-defining heterogeneities that may develop within a cell during electrochemical cycling. X-ray microscopy (XRM) is a non-destructive, advanced characterization technique that, when paired with micro-computational tomography (µCT), renders 3-dimensional images that can be utilized to analyze features with resolution dimensions as small as 700 nm, depending on sample material, thickness, and geometry. This study applied XRM with µCT to analyze Pb-acid minicells and such performance-defining heterogeneities that might develop under voltametric cycling, such as secondary phase growth and evolution, and crack and porosity formation, as well as inclusions. Utilizing ORS Dragonfly software for 3D image refinement and final stage analysis, large phase transformation was recognized for the Pb-paste within the cell between initial and final volumes, when considering secondary phase growth. Feature sizes with resolutions of 4-5 microns were observed using the NDE technique. Due to XRM’s large depth of field, this technique is considered advantageous for region of interest analysis for more in-depth studies involving Pb-based materials, that include smaller feature sizes, below submicron range, as well as for a selection stage for region of interest investigation utilizing scanning electron microscopy (SEM) or transmission electron microscopy (TEM), which was utilized for investigation of features with nm sizes.

Rechargeable lead-acid batteries have found widespread use over the past century, with applications ranging from the commonly encountered SLI (starting, lighting, ignition) batteries in motor vehicles to applications like backup power storage for critical infrastructure. The global drive to reduce dependence on fossil fuels and increase the use of renewable energies requires the development of vast amounts of reliable and safe energy storage. Lead batteries are attractive for such applications because of the high abundance of raw materials, excellent recycling of spent batteries, reliability and safety. However, current batteries suffer from limitations during deep discharge or rapid charging, which must be overcome if they are to be used for grid storage. During the discharge of lead-acid batteries, lead sulfate forms on both the negative and positive electrodes of the battery; this is a process known as sulfation. The morphology of the lead sulfate particles has a strong effect on the reversibility of this process when the battery is recharged. It is known that problems for battery life arise when coarse, needle-like lead sulfate deposits form on the electrodes of the batteries. Such deposits contribute to gradual inhibition of the battery. The electrodes in lead-acid batteries contain additives to the electroactive lead species that improve the cycling performance of the batteries. These include carbon, barium sulfate and lignosulfonates (LS), which are referred to as expanders. The presence of these compounds affects the morphology of the lead sulfate particles formed on the electrodes, and through this the ease of reversing the initial discharge reaction. LS are known to enhance discharge rates several fold, but interfere with rapid charging. However, it is not known what functional groups on the lignosulfonates play specific roles in this process. Smaller molecules that mimic specific functional groups on lignosulfonates, which are referred to as “model expander molecules” (MEMs), can be used to explore such effects in more detail. In this poster, the effect of MEMs on the morphology of PbSO4 was explored by carrying out precipitation reactions by controlled addition of Pb2+ and sulfate solutions. Scanning electron microscopy was used to evaluate differences in morphology, while phase identification and evaluation of crystallinity was carried out by power x-ray diffraction.

Lead acid battery charge and discharge rates and efficiency likely depend in part on the texture of lead sulfate crystals. That is, the distribution, size and plate-connectivity of the crystals during their formation in the discharge cycle likely influence their dissolution rates during the charging cycle. Here, we use the precipitation of a natural material, barium sulfate, as an analog to establish a fundamental basis to understand how lead sulfate texture might be affected by the rate of nucleation and growth, as well as additives to solution, such as electrolyte ions. The work may have provide some guidance on a strategy to tailor lead-acid battery composition and cycling characteristics for specific applications.
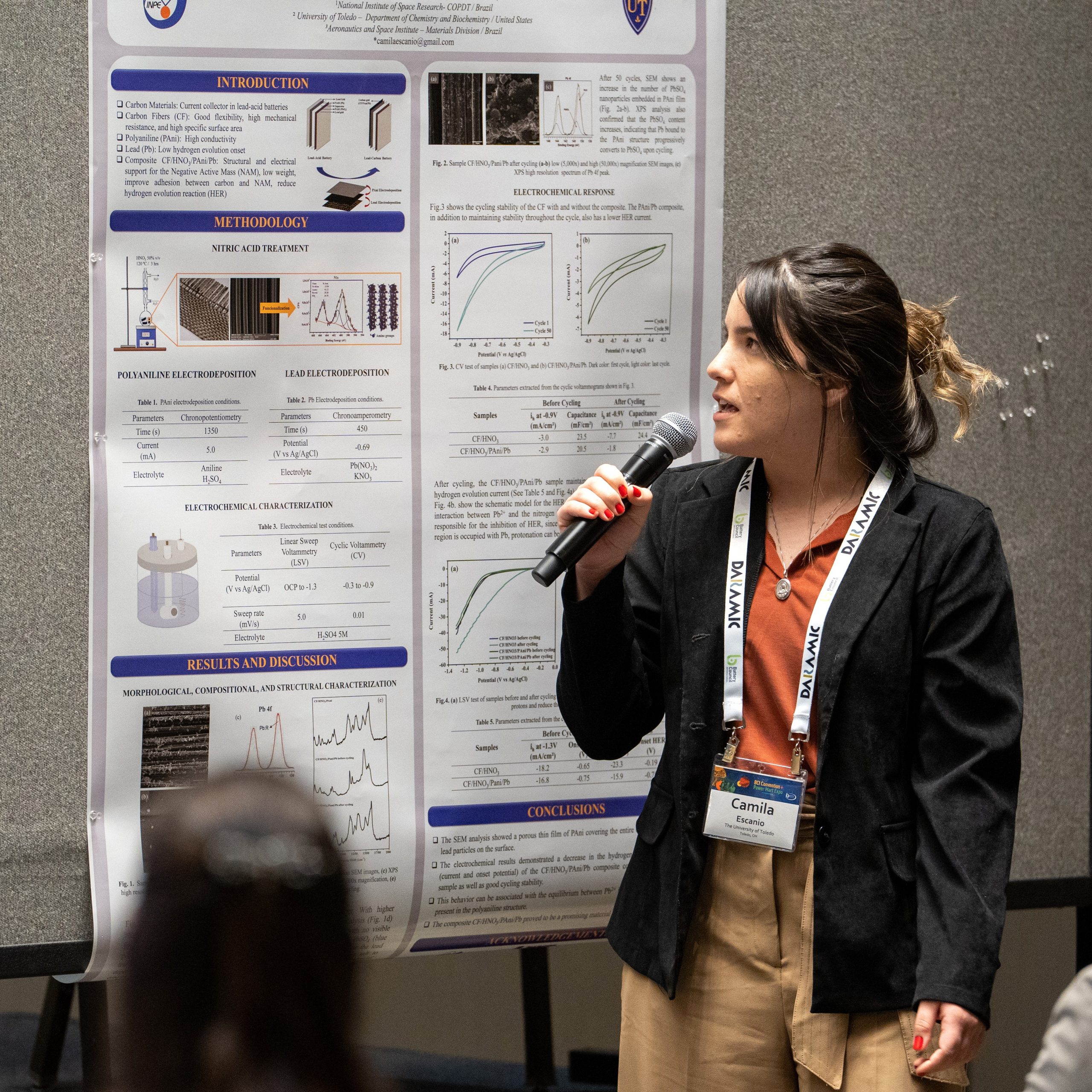
A composite made of Carbon fiber (CF), Polyaniline (PAni), and lead (Pb), with low hydrogen evolution activity, was developed and studied as a potential material to be used in lead acid batteries. First, the CF was subjected to acid treatment using 50% v/v HNO3 to remove the sizing and add nitrogen functional groups on the carbon surface (CF/HNO3). The second step, PAni electrodeposition (CF/HNO3/PAni), was performed through chronopotentiometry by applying a constant current of 5 mA for 1350 s. Next, the lead electrodeposition was carried out by chronoamperometry by applying the lead reduction potential (-0.69 V vs Ag/AgCl) for 450 s. Finally, the composite CF/HNO3/PAni/Pb was dried in a vacuum oven at 60 °C for 1 h. Morphological and structural analyses were performed using Scanning Electron Microscopy (SEM) and Raman Spectroscopy. In addition, the electrochemical behavior, such as the hydrogen evolution phenomenon and cycling stability, was evaluated by Linear Sweep Voltammetry and Cyclic Voltammetry, respectively. The SEM analysis showed a porous thin film of PAni covering the entire carbon fiber with some lead particles on the surface. Furthermore, the electrochemical results demonstrated a decrease in the hydrogen evolution phenomenon (current and onset potential) of the CF/HNO3/PAni/Pb composite compared to the CF/HNO3 sample as well as good cycling stability. Acknowledgment: The authors are grateful to FAPESP (process nº 2022/10542-4 and nº 2019/08473-1) for the financial support.

Lead batteries are only possible because of surface kinetic limitations that exist when evolving H2 over high-purity pure Pb and O2 over PbO2 electrode materials. These “gassing” reactions are secondary processes that may occur during overcharge and are highly influenced by the presence of transition metal impurities in the electrolyte, or due to the presence of Sb atoms arising from the corrosion of positive electrode plates. However, common elements present in part per million levels in secondary lead sources such as Bi, Ag, and Tl have been far explored in how they contribute to these secondary processes, particularly for the H2 evolution reaction (H2 gassing) at the negative electrode surfaces. In this study, we show how the presence of Bi, Ag, and Tl as dopants in Pb surfaces at concentrations ranging from tens to hundreds of ppm influence H2 gassing rates. Furthermore, by employing a new electrochemical in situ method that uses a stationary probe disk electrode setup (SPRDE) connected to an Inductively Coupled Plasma Mass Spectrometry (ICP-MS) instrument we were able to measure in real-time the surface dissolution processes for each dopant of interest as a function of the electrode potential. These experiments revealed selective surface dissolution from Tl atoms in the surfaces before lead discharge occurs, as well as consequent removal of traditionally more electrochemical stable elements such as Bi during lead dissolution as the first step in the discharge mechanism. We show how the removal of these trace-level impurities decreases the H2 gassing rates, but when present in the electrolyte at sufficient concentrations, the trend is reversed and H2 gassing may accelerate due to surface redeposition. Our results offer new insights into the existence of surface composition dynamics that play an important role in the secondary processes and may offer new guidelines in the design of ultra-low gassing batteries.
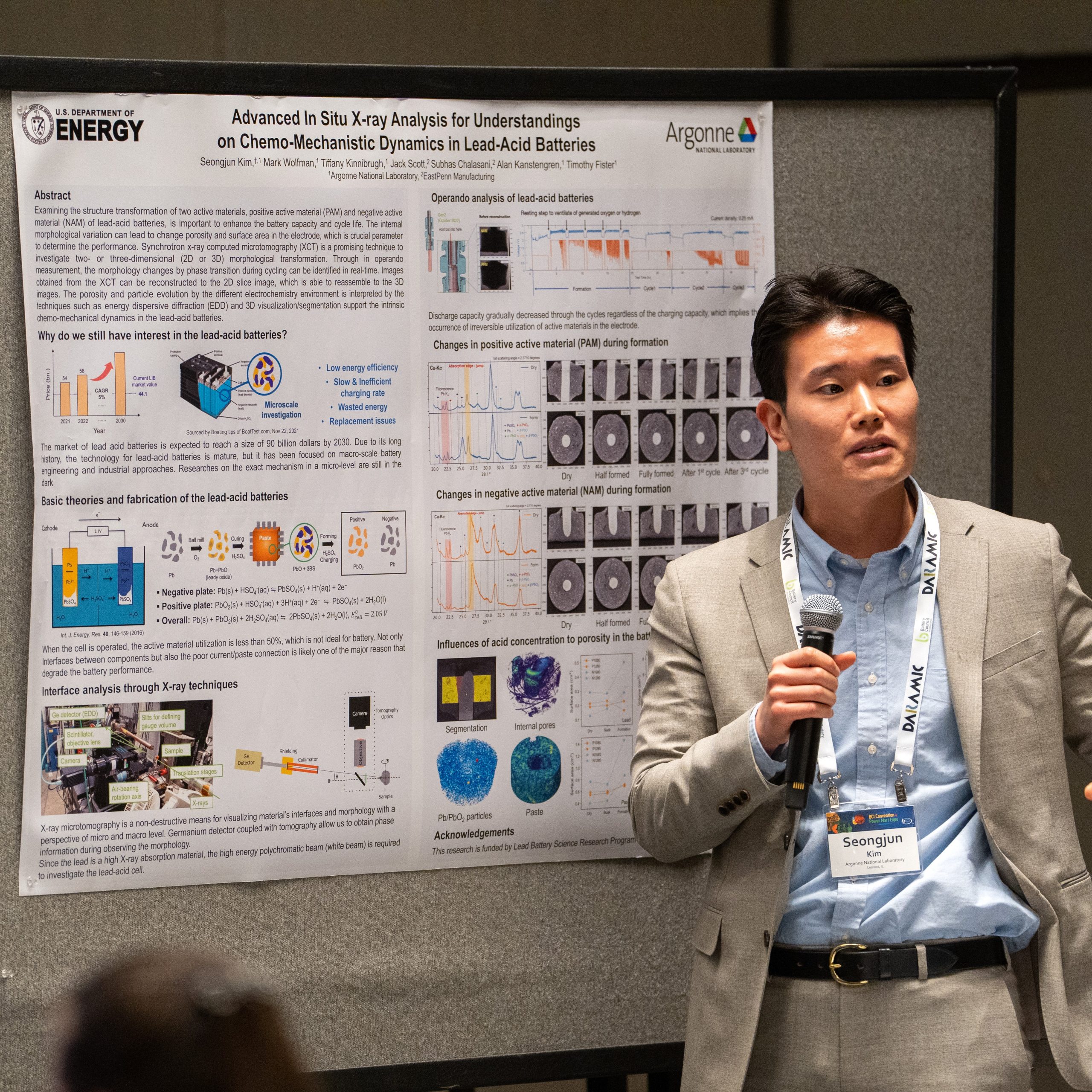
Since developed in 1859, the lead-acid batteries have been loved as secondary power source for various vehicles because of its cheap price and easy production process. Although the lead-acid battery is inferior in terms of specific energy and power density from the high atomic weight Pb compared to state-of-art lithium-ion batteries (LIB), this classical system has still loved worldwide all times because of its bold economic advantages. High powder density and simplicity of manufacture solidifies their status in the battery market. In addition, it is noteworthy that the almost every component after use can be recycled and long storage time in the perspective of energy sustainability. However, the lead-acid battery should overcome some major problems in order to compete other types of batteries. Deep discharge cause facilitation of rapid sulfation, also the irreversible crystallization of lead sulfate (PbSO4) can decrease the battery lifespan and performance. In other words, examining the structure transformation of two active materials, positive active material (PAM) and negative active material (NAM), is important to enhance the battery capacity and cycle life. The internal morphological variation can lead to change porosity and surface area in the electrode, which is crucial parameter to determine the performance. However, the existing post-mortem analysis such as scanning, and transmission electron microscopy (SEM and TEM) do not show the real-time changes during cycling. Synchrotron x-ray computed tomography (XCT) is a promising technique to investigate two- or three-dimensional (2D or 3D) morphological transformation. Through in situ or in operando measurement, the morphology changes by phase transition during cycling can be identified in real-time. Images obtained from the XCT can be reconstructed to the 2D slice image, which is able to reassemble to the 3D images. The porosity and particle evolution by the different state of charge (SOC) is interpreted by the techniques. Additionally, the other synchrotron x-ray techniques such as diffraction (XRD) and transmission x-ray microscopy (TXM) support the intrinsic chemo-mechanical dynamics in the lead-acid batteries. This presentation will provide much interest in the lead-acid batteries and information on the mechanism analysis using various synchrotron x-ray techniques. The application of in situ and operando x-ray measurements enable to enhance general comprehensions on the battery chemistry beyond not only lead-acid but other types of batteries.

Despite their long use, lead acid batteries are not fully understood. While the electrochemical properties of Pb/PbSO4 oxidation and reduction are known, the exact effects and stability of additives have not been optimized. Lignosulfonates, which are added as expanders to the negative plate, have been shown to improve discharge behavior while limiting the chargeability of lead acid batteries. However, the atomic level interactions that result in these effects are unknown. Due to the complexity of lignosulfonate structure, the functional groups responsible for the enhancements and limitations observed cannot easily be determined. It has been hypothesized that the sulfonate groups present have a significant impact on battery performance. To test the impact of sulfonates and other functional groups, simpler molecules with specific functional groups are synthesized in this work to isolate the effect of each group. The effects of these model expander molecules, or MEMs, on performance and stability can then be compared to commercially used expanders or setups without additives. MEMs were sulfonated using either sodium sulfite or sulfuric acid depending on the targeted sulfonation site. NMR and IR spectroscopy were used to verify MEM modification and purity. EDS was used to confirm the presence of sulfur and absence of inorganic byproducts. IR spectroscopy and powder diffraction were used to detect interactions with lead ions, while the stability of MEMs in 5 M sulfuric acid was tested by cyclic voltammetry. Several MEMs that were successfully synthesized showed stability in 5 M sulfuric acid as well as the ability to coordinate with lead ions.

Lead acid batteries provide a day-to-day reliable energy storage application in various fields like automotive, standby power, renewable energy, telecommunication, industrial and robotics.1 In addition to the electrochemically active lead species, these batteries contain a number of additives that improve performance and cycle life. Lignosulfonates (LS) are organic biopolymers that are used as additives in a variety application, including the production of lead acid batteries. In lead acid batteries, LS serve as organic expanders to improve the performance of the battery’s storage capacity and extending its service life, as well as by acting as a wetting agent and improving the conductivity of the electrolyte, which tends to improve battery efficiency.2 LS are molecules with high molecular weights that possess a large number of different functional groups in their structure (Figure 1). Due to the complex structural feautures of LS, it is very hard to predict the active site of functional groups interact with lead species and affect battery performance. To be able to understand the interaction of specific functional groups with lead species in detail, small molecules that mimic portions of LS can be used as model expander molecules (MEMs) (Scheme 1). In this study, we are synthesizing lignosulfonate-based MEMs. A series of MEMs were prepared from commercially available alcohols via bromination and sulfonation. The MEMs’ stability under applied potential in 5 M H2SO4 was confirmed by cyclic voltammetry. References: 1) a) G. J. May, A. Davidson and B. Monhov. Journal of Energy storage. 2018, 15, 145-157. b) R. Katahira, T. J. Elder and G. T. Beckham. Lignin Valorization: Emerging Approaches, 2018, ISBN: 978-1-78262-554-4. 2) B. O. Myrvold. Journal of Applied Electrochemistry. 2005, 35, 573-579.

The lead-acid battery is a rechargeable battery that has been in frequent use for the past century, with applications ranging from the commonly encountered SLI (starting, lighting, ignition) batteries in a motor vehicle to the less common but emerging use of lead-acid batteries for large-scale energy storage. During the discharge of lead-acid batteries, lead sulfate forms on both the negative and positive electrodes of the battery; this is a process known as sulfation. When the lead sulfate that builds up consists of individual particles that are fine and uniform in nature, this process is reversible when the battery is recharged. Problems for battery life arise when coarse, needle-like lead sulfate deposits form on the electrodes of the batteries. Such deposits contribute to gradual inhibition of the battery. The electrodes in lead-acid batteries contain additives to the electroactive lead species that improve the cycling performance of the batteries. These include carbons, barium sulfate and lignosulfonates, which are referred to as expanders. The presence of these compounds affects the morphology of the lead sulfate particles formed on the electrodes, and through this the ease of reversing the initial discharge reaction. However, it is not known what functional group on the lignosulfonates may play a role in this process. Smaller molecules that mimic specific functional groups on lignosulfonates, named “model expander molecules” (MEMs) can be used to explore the effects in detail. In this poster, the precipitation of lead sulfate using controlled addition of Pb2+ to sulfate solutions in the absence and presence of these MEMs was explored. Scanning electron microscopy was used to evaluate differences in morphology of lead sulfate precipitated under a variety of different conditions. Phase identification and evaluation of crystallinity was carried out by power x-ray diffraction.

As particles get smaller, their surface area to volume ratio rises. The multi-molecular adsorption technique “BET”, to determine the surface area was invented by Stephen Brunauer, Paul Hugh Emmett, and Edward Teller in 1938. Brunauer, Emmett, and Teller expanded Langmuir’s (1918) adsorption to several molecular layers to understand how adsorption data affects material attributes such as total surface area, pore-size distribution, micro-pore analysis, and porosity. BET method is used for both macro and nano materials and is a common technique used to classify raw materials and active materials in the battery industry. This study mainly concentrates on finding the correlation coefficient for each ingredient of a lead-acid battery paste mix against BET surface area values. This is done by developing a master data matrix that collects weight percentages of the ingredients in a lead acid battery paste mix with respect to lead-oxide content. The BET surface area results of the dry cured battery active material are then compared to the weight percentages of each ingredient to determine that ingredient’s effect on surface area. Correlation coefficients measure linear relationships between variables. -1 to 1 are its values. According to a correlation value of -1, one series rises while the other falls, and vice versa. 1 is a perfect positive connection. Zero correlation means no linear relationship.

Lead batteries have the economic potential to continue serving as an important energy storage technology for a decarbonized sustainable energy future. Advancing the design of lead batteries with higher material utilization, fast recharge rates, and long-cycle life requires a fundamental understanding of the electrochemical and chemical processes taking place at the atomic and molecular scales. In this work, we will discuss how the use of well-defined lead negative electrode interfaces allows us to gain insights into the fundamental limits of discharge capacity and recharge rates. By starting from ultra-high purity, clean, and electropolished smooth lead surface with nanometer-scale surface roughness we were able to unveil the relationships between discharge rates and PbSO4 particle size/layer thickness that ultimately governs the maximum discharge capacity accessible as a function of discharge rate. This data helped us develop a mathematical model that captures the key processes concerning lead ion and (bi)sulfate ion gradients coupled to nucleation and growth that explain, from first principles, the origin of the well-known empirical Peukert law. In this context, exploring how variables such as acid concentration, temperature, and the presence of lignosulfonate additives in the electrolyte further influence discharge capacity allow us to understand how thermodynamic and kinetic parameters play a huge role in governing the accessible capacity from negative electrodes. This study paves the way to a deeper understanding of the variables that are most important in improving the discharge capacity of negative electrodes, such as the design of expander molecules.

Understanding the factors governing the recharge process of negative electrodes in lead batteries may open new opportunities in the design of advanced batteries for automotive applications as well as for grid energy storage systems. The conversion of lead sulfate back to metallic lead is a complex process that may involve chemical dissolution followed by electrochemical reduction of sparingly soluble lead ions at the negative electrode surface. Depending on the discharge conditions, rest periods, and overall battery operational protocol, lead sulfate crystals will vary in size and thus influence recharge rates. Here, we employed a well-defined electrochemical interface approach to gain insights into the recharge mechanism and understand the fundamental kinetic limits of the charging process. By using thickness-controlled lead sulfate layers prepared over lead surfaces and charging the electrode using a potentiostatic scan we were able to reveal how the kinetics of the chemical dissolution step from PbSO4 to Pb2+ ions is the rate-determining step of this reaction. In turn, this allows us to define a new metric called kinetic charge acceptance (KCA) that establishes how fast one can recharge a lead sulfate layer of a given layer thickness or equivalent particle size. Furthermore, we provide direct experimental evidence of how lead sulfate particle size distribution governs the recharge rates by using chemically synthesized PbSO4 particles with sizes ranging from 50 nm up to 1 micrometer deposited on carbon electrodes. The use of well-defined particles allowed us to observe an inverse relationship between particle size and recharge rates, namely, an increase in lead sulfate average particle size from 100 nm to 1000 nm decreases the measured KCA by a factor of 10. These results indicate the importance that controlling the lead sulfate particle size during discharge can have in the overall negative electrode rechargeability, and it also opens new opportunities to improve the formation of batteries during manufacturing.
By connecting key stakeholders at domestic manufacturers with the world-class research capabilities at our national labs and the Department of Energy, we can accelerate research timelines and commercialize new products efficiently and cost-effectively.